Performance Evaluation of Clinical Detection of Coagulation Factors, Protein S, Protein C, Anti-thrombin Ⅲ, and von Willebrand Factor Antigen
-
摘要:目的 对血栓与止血特殊项目的临床检测进行全面性能评价, 以期为临床提供可靠的检测结果。方法 按照美国临床实验室标准化协会(Clinical and Laboratory Standards Institute, CLSI)EP5-A2、EP9-A2文件及《卫生部临床血液学检验常规项目分析质量要求WS/T 406-2012文件》标准, 对本实验室ACL TOP 700全自动凝血分析仪测定凝血因子(FⅡ、FⅤ、FⅦ、FⅧ、FⅨ、FⅩ、FⅪ、FⅫ)、易栓症筛查三项(蛋白S、蛋白C及抗凝血酶-Ⅲ)和血管性血友病因子抗原(von Willebrand factor antigen, vWF:Ag)共12个项目的准确度、不精密度、携带污染率、线性、方法学比对及参考范围进行全面性能评估。结果 美国病理学家协会提供的12个项目质评物检测结果与靶值相比, 除蛋白C低值外偏差均小于15%。8个凝血因子批内不精密度为1.7%~4.4%, 均小于4.5%;批间不精密度为2.2%~7.5%, 均不大于7.5%。易栓症筛查三项的批内不精密度为1.0%~7.0%;批间不精密度为1.5%~10.5%。vWF:Ag批内不精密度为2.2%~3.1%, 批间不精密度为2.1%~4.1%。12个项目携带污染率为0~2.63%, 均小于3%。线性验证实验结果显示蛋白S、蛋白C、抗凝血酶-Ⅲ及vWF:Ag相关系数为0.982~0.988, 均≥0.975。ACL TOP 700与参比检测系统进行比较, 12个项目检测结果的相关系数均≥0.975, 一致性较好。厂商提供的参考范围均适用于本实验室。结论 ACL TOP 700全自动凝血分析仪对血栓与止血特殊项目检测的准确度、不精密度、携带污染率、现行线性范围及参考范围验证等性能评价良好, 适用于临床标本检测。Abstract:Objective To conduct a comprehensive performance evaluation of fully automated coagulation analyzer in testing coagulation factors, protein S(PS), protein C(PC), anti-thrombin Ⅲ (AT-Ⅲ), and von Willebrand factor antigen (vWF:Ag).Methods According to the Clinical and Laboratory Standards Institute (CLSI) EP5-A2, EP9-A2, and WS/T 406-2012 specifications, coagulation factors(FⅡ, FⅤ, FⅦ, FⅧ, FⅨ, FⅩ, FⅪ, and FⅫ), PS, PC, AT-Ⅲ, and vWF:Ag were detected in the apparatus to evaluate the accuracy, within-run and between-run imprecisions, linear range, carryover rate, method comparisons, and reference range of the ACL TOP 700 coagulation analyzer.Results The instrument had high accuracy and precision, a good linear range, and low carryover rate (0-2.63%, < 3%). The biases of the 12 College of American Pathologists (CAP) controls compared with the target value were all less than 15%, excepting the low value of PC. The within-run imprecisions of 8 coagulation factors were 1.7%-4.4%, and the between-run imprecisions were 2.2%-7.5%. The within-run imprecisions of 3 thrombophilia screen tests and vWF:Ag were 1.0%-7.0% and 2.2%-3.1%, and he between-run imprecisions were 1.5%-10.5% and 2.1%-4.1%, respectively. The carryover rates of the 12 items ranged from 0 to 2.63%. Results of liner verification test showed the correlation coefficients of PS, PC, AT-Ⅲ, and vWF:Ag were 0.982-0.988. Results of method comprisons showed the correlation coefficients of ACL TOP 700 and other coagulation analyzer were more than 0.975, and the recommended reference ranges of all the 12 items were appropriate for our laboratory.Conclusions ACL TOP 700 coagulation analyzer has a good performance in accuracy, imprecision, carryover rate, linear range, and method comparison. It is suitable for detection of clinical specimens.
-
Keywords:
- coagulation analyzer /
- thrombosis and homeostasis /
- performance
-
妊娠期高血压疾病(hypertensive disorders of pregnancy, HDP)是以妊娠期血压异常升高为主要特征的一组疾病,是全世界范围内造成孕产妇和胎儿死亡的主要原因之一[1-2]。HDP病因复杂,详细机制尚不明确,早识别高危患者并及时予以恰当干预是改善预后的关键环节。目前国内外多个临床中心研发了HDP不良结局的预测模型[3-4],但新模型在应用于临床实践前须对其有效性及普适性进行验证,且要求其预测因子最好为临床常规筛查指标,以增强模型在临床应用的可及性。本研究基于苏州大学附属第一医院及四川省妇幼保健院两所医疗机构连续8年HDP患者的临床数据,对国内外文献报道的6种HDP预测模型在中国不同地区人群中应用的临床价值进行分析,以期为进一步建立适合中国国情的预测模型提供参考依据。
1. 资料与方法
1.1 研究对象
本研究为回顾性分析。研究对象为2011年5月1日至2019年4月30日于苏州大学附属第一医院、四川省妇幼保健院分娩(孕周≥28周)且入院诊断为HDP的所有患者。HDP的诊断标准符合中华医学会制定的《妊娠期高血压疾病诊治指南(2020)》[5]。排除标准:病史资料不完善以及预测指标数据收集前已出现临床结局的病例。
本研究已通过苏州大学附属第一医院(审批号:2020-099)、四川省妇幼保健院(审批号:2020-1113-123)伦理审查委员会审批,并豁免患者知情同意。
1.2 方法
1.2.1 文献检索及模型介绍
以“hypertensive disorders of pregnancy”“pree-clampsia”“prediction model”“prognosis”“adverse outcomes”“妊娠期高血压疾病模型”“子痫前期模型”“不良结局”“预后”等关键词相组合的方式,检索PubMed、Medline、Embase等英文数据库以及中国知网、万方数据知识服务平台等中文数据库,共获取14种HDP预后相关预测模型[6-19]。鉴于回顾性研究数据收集的局限性,要求模型的预测参数为临床常规检测指标,经筛选后保留其中6种预测模型[8-9, 11-12, 15, 17], 详见表 1。
表 1 本研究选取待验证的6种HDP不良结局预测模型模型 发表时间 预测公式 AUC(95%CI) fullPIERS[8] 2011年 logit(P)=2.68-0.0541×(孕龄)+1.23×(胸痛或呼吸困难)-0.0271×(Cr)+0.207× (PLT) +4.00×10-5×(PLT)2+0.0101×(AST)-3.05×10-6× (AST)2+2.50×10-4×(Cr)× (PLT)-6.99×10-5×(PLT)×(AST)-2.56× 10-3×(PLT)×(SpO2) 0.88(0.84~0.92) miniPIERS[9] 2014年 logit(P)=-5.77-0.298 ×(经产妇)-1.07× log(孕龄) + 1.34× log(收缩压) -0.218× (随机尿蛋白++) + 0.424 × (随机尿蛋白+++) +0.512× (随机尿蛋白++++) +1.18×(阴道出血伴腹痛)+ 0.422 ×(头痛或视觉障碍)+0.847 × (胸痛或呼吸困难) 0.768(0.735~0.801) Zwertbroek[11] 2017年 logit(P)=-2.036-0.086×(年龄)+0.418×(合并症)+0.863 ×(慢性高血压)-0.135×(孕周)+0.045 ×(收缩压)-0.004×(PLT)+0.015×(Cr)+0.003×(LDH)+0.570×ln(存在蛋白尿,即PCR≥30 mg/mmol或24 h尿蛋白定量≥300 mg) 0.760(0.731~0.807) PREP[12] 2017年 PREP-L(从诊断早发型PE至产后出院期间整体不良结局风险评估):
Probability (P) =exp(X)/[1 + exp(X)]
X=-1.507-0.020×(年龄) + 12.052×{[log(孕周)]3-39.902 41}-7.930×{[log(孕周)]3×log[log(孕周)]-49.081 88}-0.330× (存在一项既往史)-0.579×(存在两项及以上既往史)+ 0.146×log(PCR)-0.951×[log(Ure)-1]-0.004×(PLT)+ 0.024×(收缩压)+0.409×(使用降压药)+1.252×(使用硫酸镁)
PREP-S(从诊断早发型PE至孕34周期间不同时间点生存率评估):
S(t)=S0 (t)§ ^exp [(β1×X1 + …+βn×Xn)]
S(t)=S0(t)^exp(K)
K=-0.031×(年龄)+1.514×{[log(孕周/10)]-2-0.834 513 6}+ 5.707×{[log(孕周/10)]-2×ln[log(孕周/10)]-0.065 215 5}+ 0.122(腱反射亢进)-0.169×(存在一项既往史)-0.384×(存在两项及以上既往史)+ 0.016×(收缩压)+0.797×(SpO2<94%)-0.002×(PLT)+ 0.126×log(ALT)+ 0.605×log(Ure)2-0.144×log(Ure)3+0.265×log(Cr)+ 0.080×log(PCR)+ 0.176×(使用降压药)+ 1.066×(使用硫酸镁)
§S0 (t)-基线生存调整为适合的时间t
S0(48 h)=0.991 42, S0(72 h)=0.985 42, S0(1周)=0.964 92,
S0(1个月)=0.873 77PREP-L:
0.82 (0.80~0.84)
PREP-S:
0.84 (0.81~0.87)Ngwenya[15] 2020年 logit(P)=1.549 -0.041×(年龄)-0.044 ×(孕周)+ 0.133×(上腹部疼痛) +0.39 ×(阴道出血伴腹痛)-0.135 ×(Hb)-0.160×(PLT) 0.796(0.758~0.833) 马国珺[17] 2020年 logit(P)=-2.846+2.344×(蛋白尿)+1.054×(肝功能损伤)+1.116×(血小板减少症)+1.254×(早发型PE) 0.754(0.720~0.789) HDP: 妊娠期高血压疾病;AUC:曲线下面积(原文献中);Cr:肌酐;PLT:血小板计数;AST:谷草转氨酶;ALT:谷丙转氨酶;SpO2:脉搏血氧饱和度;LDH:乳酸脱氢酶;PCR:尿蛋白肌酐比;Hb:血红蛋白;Ure:尿素;PE:子痫前期 1.2.2 资料收集及不良结局定义
基于病案室的病历数据系统收集6种预测模型涉及的患者临床资料。本研究中的“入院”是指终止妊娠时的入院。孕产妇特征及临床表现相关资料均源于首次病程记录,除24 h尿蛋白定量为入院48 h内的检验结果外,其余检验指标均为入院24 h内的结果。若患者该期间存在多次测量记录,以首次检验结果为准。
HDP患者不良结局的定义参照既往文献基于Delphi法达成的共识[8, 12],包括孕产妇不良结局和围产儿不良结局。(1)孕产妇不良结局:死亡、中枢神经系统疾病(子痫、格拉斯哥昏迷评分<13分、卒中或可逆性缺血性神经系统障碍、皮质盲或视网膜剥离、一过性缺血和迟发型可逆性脑病等),心血管和呼吸系统疾病[接受强心治疗、心肌缺血或梗死、SpO2<90%、需高浓度吸氧(氧浓度≥50%)超过1 h、除剖宫产外的插管、肺水肿和不能控制的严重高血压等],血液系统疾病(输注血制品、输血前血小板计数<50 ×109/L),肝脏疾病(肝血肿或破裂),肾脏疾病(急性肾功能不全:既往无肾病史者肌酐>150 μmol/L、既往有肾病者肌酐>200 μmol/L或进行透析),弥散性血管内凝血,胎盘早剥,产后出血需输血或栓塞或子宫切除,HELLP综合征等。(2)围产儿不良结局:孕<34周早产、死胎等。本研究规定上述不良结局事件中,发生任意一项即认为发生了不良结局。本研究以入院48 h为研究的时间窗,主要基于以下考虑:该时间段内对于治疗方案的制订具有重要临床意义,可为注射糖皮质激素促进胎儿成熟、转诊等措施的采取争取时间。
1.3 统计学处理
采用SPSS 24.0软件进行统计学分析。年龄、孕前体质量指数符合正态分布,以均数±标准差表示。不良结局为计数资料,以频数(百分数)表示。从区分度和校准度两个方面对模型的预测能力进行评估。其中区分度以受试者操作特征(receiver operating characteristic, ROC)曲线下面积(area under the curve, AUC)表示(AUC>0.7表示区分度良好),并采用Delong检验对不同模型的AUC进行比较。基于约登指数获取预测模型的最佳临界值,并计算灵敏度、特异度、阳性似然比和阴性似然比。采用Hosmer-Lemeshow拟合优度检验评估模型的校准度,若该检验的P>0.05,表示拟合值与观测值的吻合程度较一致,模型的拟合性较好。以P<0.05为差异具有统计学意义。
2. 结果
2.1 一般临床资料
共入选符合纳入和排除标准的HDP患者2978例,包括苏州大学附属第一医院1492例、四川省妇幼保健院1486例。研究对象入选流程见图 1。
2978例HDP患者,平均年龄(30.1±5.0)岁,平均孕前体质量指数(22.7±3.7)kg/m2,入院孕周为37.1周(范围:35.6~39.0周)。初次妊娠1096例(36.8%),多胎妊娠185例(6.2%),有流产史1553例(52.1%),有生育史1084例(36.4%)。合并慢性高血压342例(11.5%),免疫性疾病37例(1.2%),慢性肾病39例(1.3%),孕前糖尿病37例(1.2%),妊娠期糖尿病714例(24.0%)。有HDP史153例(5.1%),有子痫前期史65例(2.2%)。
2.2 不良结局
2978例HDP患者,住院期间共655例(22.0%)发生不良结局事件,其中405例(13.6%)发生于入院48内。孕<34周分娩(49.4%, 200/405)、需输血治疗(43.5%, 176/405)、胎盘早剥(23.5%, 95/405)是入院48 h内最常见的不良结局事件。
2.3 预测模型性能评估
基于病历系统获取的数据,根据表 1中的公式计算不良结局发生风险,并对6种模型的预测能力进行验证。ROC曲线显示,6种模型(PREP模型包括PREP-L与PREP-S)预测HDP患者入院48 h内/住院期间发生不良结局的AUC分别为0.711、0.723、0.739、0.897(PREP-L)与0.745(PREP-S)、0.600、0.729。Delong检验显示,fullPIERS与mini-PIERS模型的AUC差异无统计学意义(P=0.552),Ngwenya模型的AUC低于马国珺模型(P<0.001)。鉴于PREP模型的验证人群较少,故未对该模型进行校准度评估。Hosmer-Lemeshow检验显示,余5种模型的P值均小于0.05,提示模型的拟合性较差。结合区分度和校准度评估结果可知,除Ngwenya模型外,余5种模型对HDP患者不良结局均具有一定预测效果,但模型的拟合性欠佳,整体预测性能不高(表 2,图 2)。
表 2 6种模型预测HDP患者不良结局的预测价值模型 验证人群 验证人数
(n)入院48 h内发生不良结局患者
[n(%)]预测入院48 h内发生不良结局的AUC(95% CI) 最佳临界值
(%)灵敏度
[%(95% CI)]特异度
[%(95% CI)]阳性似然比
(95% CI)阴性似然比
(95% CI)fullPIERS HDP患者 2978 405(13.6) 0.711
(0.682~0.739)1.9 65.9
(61.1~70.5)65.9
(64.0~67.7)1.93
(1.8~2.1)0.52
(0.5~0.6)miniPIERS HDP患者 2978 405(13.6) 0.723
(0.692~0.754)1.1 64.4
(59.6~69.1)76.6
(74.9~78.2)2.75
(2.5~3.0)0.46
(0.4~0.5)Zwertbroek 孕34+0~36+6周HDP患者 580 95(16.4) 0.739
(0.680~0.797)29.1 69.5
(59.2~78.5)71.1
(66.9~75.1)2.41
(2.0~2.9)0.43
(0.3~0.6)PREP* PREP-L 孕<34周PE患者 21 17(81.0)† 0.897
(0.754~1)§- - - - - PREP-S 孕<34周PE患者 21 11(52.4) 0.745
(0.528~0.963)- - - - - Ngwenya 重度PE患者 1248 326(26.1) 0.600
(0.562~0.638)22.6 57.1
(51.5~62.5)60.1
(56.8~63.3)1.43
(1.3~1.6)0.71
(0.6~0.8)马国珺 重度PE患者 1248 326(26.1) 0.729
(0.696~0.761)63.5 66.3
(60.8~71.4)70.0
(66.9~72.9)2.21
(1.9~2.5)0.48
(0.4~0.6)HDP、PE、AUC:同表 1;†住院期间发生不良结局的患者数及其所占比例;§住院期间发生不良结局的AUC(95%CI);*鉴于PREP模型的验证人数较少,未检验其灵敏度、特异度、阳性似然比、阴性似然比 ![]() 图 2 6种模型预测HDP患者不良结局的受试者操作特征曲线HDP:同表 1
图 2 6种模型预测HDP患者不良结局的受试者操作特征曲线HDP:同表 13. 讨论
HDP可导致多种不良结局事件,严重危害母婴安全。鉴于HDP病因涉及多方面因素,多个通路和多种机制参与其发病,目前尚缺乏有效的干预措施,及时预测不良结局发生风险并提前干预,对预防HDP不良结局发生具有重要意义。目前,国内外学者已针对HDP不良结局研发了相应预测模型,且随着研究的深入,预测参数也从传统的单一指标逐渐向多指标、多元化转化。鉴于不同模型适用的人群不同,且HDP发病受多种因素的影响,预测模型在不同地区人群中应用时仍需进行外部验证。本研究对国内外研发的6种预测模型在中国不同地区人群的适用性进行了分析,结果显示6种模型预测HDP患者入院48 h内/住院期间发生不良结局的AUC分布于0.600~0.897,除Ngwenya模型外,其余模型的区分度均良好(AUC>0.7),但模型的拟合性差,整体预测性能不高。
2011年von Dadelszen等[8]在一项前瞻性研究中纳入了2023例子痫前期(preeclampsia,PE)患者,并建立白种人群不良结局的预测模型(fullPIERS),该模型综合了孕周、临床症状、肝肾功能以及其他血液学指标的影响,预测PE患者入院48 h内出现不良结局的AUC高达0.88。基于该模型可将患者分为低风险(风险值<2.5%)、高风险(风险值≥30%)人群,从而更有效地对患者进行管理。国内外其他学者针对该模型已进行了外部验证,总体预测价值较好[3]。本研究对该模型在中国东西部地区HDP人群应用情况进行了性能评价,发现其预测HDP患者入院48 h内发生不良结局的AUC仅为0.711;低风险人群中不良结局发生率为8.2%(172/2099),略高于原始研究(1%),而高风险人群中有94.4%(17/18)发生了不良结局,远高于原始研究(59%)。不同研究之间该模型的预测效果差异显著,可能与该模型是基于白色人种建立,黄色人种在人口学特征上可能与白色人种存在差异,且HDP不良结局发生率受该地区经济和医疗水平等因素影响有关。von Dadelszen等[8]指出,该模型仅适用于有良好医疗体系、可及时正确处理该类孕产妇的高收入国家/地区或医疗机构,而在中低收入国家使用时可能受限。本研究的两所医疗机构虽均位于经济相对发达地区,且均为危重症孕产妇转诊中心,但相对于发达国家而言,医疗保障体系欠完善,收治的转院患者尤其病情危重者多数未经正规产检和规范化治疗,可能在一定程度上解释了相同的风险值在本研究数据中显示出不良结局发生风险明显增高的现象。此外,本研究团队既往已针对PE患者对该模型进行了验证,结果表明其预测价值较好[20]。本研究纳入了更广泛的HDP患者,且包含多中心数据,虽然其预测不良结局的AUC有所降低,但预测性能仍良好。需注意的是,不管原文报道还是本研究结果均提示该模型的预测参数SpO2易缺失。临床并未将该指标纳入常规检测,仅出现胸闷等不适时才进行监测。根据文献中的意见,该项因子缺失时以97%作为替代值进行预测,因此该模型的准确性和适用性尚有待评估。
目前,孕产妇和围产儿死亡大多发生于中低收入国家[21]。miniPIERS是基于5个中低收入国家共2081例HDP患者临床数据建立的模型[9],该模型预测入院48 h母婴预后不良的AUC为0.768,识别的高风险孕产妇可从硫酸镁、抗高血压药物、糖皮质激素或转诊等干预措施中获益。本研究该模型预测HDP患者入院48 h内出现不良结局的AUC为0.723,与原始文献报道结果较接近。虽然该模型建立者Payne等[9]建议预测风险值>15%时应引起临床重视,但本研究未观察到风险值>15%的病例,可能是由于miniPIERS模型主要基于患者基本信息及临床症状相关资料所建立,而本研究病例来源于国内经济较发达地区的医疗机构,产前保健系统相对完善,许多患者在未出现明显临床症状前已收治入院并予以干预,以致未发现风险值>15%的人群。因此,很难单独将母体信息及临床表现作为早期预警方式,其临床适用性不高。
Ngwenya亦是基于中低收入国家(津巴布韦)人群(n=549)建立的预测模型[15],该模型由年龄、孕周、上腹部疼痛、阴道出血伴腹痛、血红蛋白、血小板计数6个参数构成,经ROC曲线验证其预测重度PE患者预后不良的性能良好(AUC:0.796)。与miniPIERS模型不同,该模型纳入了血液学指标(血红蛋白和血小板计数),这是考虑到妊娠期贫血是大多数发展中国家孕产妇发生重度PE、死亡以及新生儿预后不良的主要原因之一[22]。本研究结果显示,该模型预测HDP患者入院48 h内发生不良结局的AUC不足0.7,提示该模型在中国东西部地区HDP人群中的适用性较低。
Zwertbroek模型源于HYPITAT-Ⅱ试验中的519例HDP患者二次数据分析,是对终止妊娠风险进行的预测,包括进展为严重并发症、HELLP综合征、肺水肿及子痫抽搐等[11],该模型可将孕34~37周罹患严重疾病高风险与低风险孕妇相区分(AUC:0.760)。根据模型预测结果,Zwertbroek模型将风险值≤22%人群定义为低风险组,仅常规监测即可;风险值在22.1%~44.4%为中风险组,考虑频繁监测或促子宫颈成熟;风险值≥45%者为高风险组,可考虑立即终止妊娠。本研究中该模型预测HDP患者入院48 h内发生不良结局的AUC为0.739;低风险人群中,8.9%(27/303)发生不良结局;高风险人群中,40.9%(45/110)发生不良结局,提示可根据模型识别结果及早发现终止妊娠高风险个体。国际上亦有随机对照试验探讨了该时段孕周期待管理与有计划早期分娩人群母婴结局的差异性,试图寻找孕产妇最佳分娩时机。Chappell等[23]研究发现,相较于期待管理,早期分娩可显著降低孕产妇的不良结局发生风险,且未见新生儿发病率有明显提高。因此,针对高风险孕妇终止妊娠时需充分权衡母婴获益与风险。此外,该模型提示合并慢性高血压是不良结局的危险因素,临床需增加对此类孕妇的关注度,可于早孕期使用阿司匹林,以预防或延缓高危人群发生PE及相关并发症[24-26]。
鉴于早发型和晚发型PE的病因和发病机制不同, Thangaratinam等[12]在一项纳入946例早发型PE患者的前瞻性研究中建立了PREP模型,该模型从PREP-L和PREP-S两个方面对早发型PE患者的预后进行预测,前者是对从诊断早发型PE至产后出院整体不良结局的风险评估,后者是从诊断早发型PE至孕34周不同时间点生存率的评估。经外部数据集验证,在当前护理条件下PREP模型可用于早发型PE患者早期早产、入院48 h前和入院期间不良结局风险的预测。本研究结果显示,PREP-L和PREP-S预测孕<34周PE患者住院期间预后不良的AUC均达到良好水平,尤其PREP-L模型,AUC(0.897)远高于其他模型。但由于该模型仅针对早发型PE患者,且本研究选取的医疗机构在此研究期间并未将尿蛋白肌酐比作为常规筛查指标,以致大量病例被排除,最终导致样本量不足(仅21例),因此该模型的预测价值仍需验证。
马国珺等[17]基于2015年1月至2018年12月全国4家医院817例重度PE患者的临床资料建立了此类人群母胎不良结局的预测模型(AUC:0.754),并提出临床可重点监测蛋白尿、肝功能、血小板水平,以有效识别高危患者。本研究亦表明,该模型对重度PE患者入院48 h内不良结局具有良好的预测效能(AUC:0.729)。但该模型涉及的预测因子中,24 h尿蛋白定量(≥300 mg)在许多医疗机构并非常规检测项目,且该指标的收集较为繁琐,患者依从性差,导致测定结果不准确。本研究约18.8%(234/1 248)重度PE患者缺乏24 h尿蛋白定量数据(模型验证时以随机尿蛋白阳性为蛋白尿阳性),故该模型大范围开展的可行性有待商榷。
本研究为目前在中国地区对HDP患者预后模型进行外部验证样本量最大的双中心研究。纳入的两所医疗机构分别位于中国东、西部地区,兼顾了病例来源的地域差异,以更好地评估模型的普适性。本研究局限性:(1)回顾性分析难以避免信息偏倚。某些预测因子如尿蛋白肌酐比并非常规筛查指标,以致对PREP模型[12]验证时样本量较少。(2) 鉴于一些预测因素在回顾性研究中不易获得,如胎盘生长因子、可溶性fms样酪氨酸激酶1等[18-19, 27]需专门的实验室检测,临床可及性差,本研究仅对预测参数相对易获得的6种模型性能进行了比较。(3)为增加研究结果的临床意义,验证人群未全部采用与原始研究相同的定义和数据采集方法进行纳入,而采取了广义的外部验证模式,纳入更广泛的患者。如在fullPIERS模型中,原始研究的验证人群为PE患者,本研究为HDP患者。
综上,6种模型对中国东西部地区HDP患者不良结局均具有一定预测能力,其中以fullPIERS、miniPIERS、Zwertbroek、PREP及马国珺模型的应用价值相对较高,但部分模型的预测指标非常规检查项目,实际应用时需结合本地经济状况、医疗水平以及模型预测参数的可行性综合考虑。此外,由于本研究验证模型的校准度均较差,可能与多数模型是基于国外人群所建立,国内外人群在种族、数据分布特征以及经济水平等方面存在差异有关。在大数据时代下,通过多中心协作,建立适合我国国情甚至局部地区的HDP患者不良结局预后模型具有必要性,以更有效地指导临床工作,对孕产妇进行管理,这将为降低我国孕产妇及围产儿死亡率奠定重要基础。
-
表 1 凝血因子、易栓症筛查三项和vWF : Ag准确度结果
项目 CGE1 CGE2 靶值 测定值 偏差(%) 靶值 测定值 偏差(%) 凝血因子 FⅡ 93.4 85.4 -8.57 35.5 33.2 -6.48 FⅤ 91.2 81.4 -10.75 33.6 31 -7.74 FⅦ 101.9 92.7 -9.03 34.5 31.7 -8.12 FⅧ 105.7 104.1 -1.51 163.2 166.4 1.96 FⅨ 115.7 117.4 1.47 45.7 47.9 4.81 FⅩ 97 89.4 -7.84 35.1 33.8 -3.7 FⅪ 88.3 93.8 6.23 36.4 38.2 4.95 FⅫ 101.8 97.2 -4.52 38.4 36 -6.25 易栓症筛 PS 93.4 101.1 8.24 32.1 28.5 -11.21 查三项 PC 97.5 85 -12.82 31.3 26 -16.93 AT-Ⅲ 86.6 91 5.08 30.4 31.1 2.3 vWF:Ag 158.2 167 5.56 240.7 214.8 -10.76 PS:蛋白S;PC:蛋白C;AT-Ⅲ:抗凝血酶-Ⅲ;vWF : Ag:血管性血友病因子抗原 表 2 凝血因子八项批内不精密度及批间不精密度
项目 批内不精密度 批间不精密度 水平1(x±s) CV(%) 水平2(x±s) CV(%) 水平1(x±s) CV(%) 水平2(x±s) CV(%) FⅡ 29.2±0.61 2.1 84.8±2.58 3.0 31.61±1.94 6.1 93.27±4.52 4.8 FⅤ 27.3±0.94 3.5 83.1±1.87 2.3 30.12±1.90 6.3 103.55±7.46 7.1 FⅦ 25.8±0.58 2.2 85.0±1.76 2.1 27.17±2.05 7.5 98.58±3.35 3.4 FⅧ 24.8±0.61 2.5 79.3±2.86 3.6 24.74±1.83 7.4 72.47±3.42 4.7 FⅨ 37.6±1.65 4.4 112.3±45.2 4.0 37.26±1.85 5.0 99.13±6.15 6.2 FⅩ 29.1±0.93 3.2 88.1±1.49 1.7 32.66±2.25 6.9 95.49±2.14 2.2 FⅪ 31.5±1.06 3.4 91.0±3.90 4.3 32.21±1.36 4.2 97.30±6.40 6.6 FⅫ 28.6±0.86 3.0 83.3±1.58 1.9 28.84±1.30 4.5 83.06±3.77 4.5 CV:变异系数 表 3 易栓症筛查三项及vWF : Ag批内不精密度及批间不精密度
项目 批内不精密度 批间不精密度 水平1(x±s) CV(%) 水平2(x±s) CV(%) 水平3(x±s) CV(%) 水平1(x±s) CV(%) 水平2(x±s) CV(%) 水平3(x±s) CV(%) PS 25.38±1.78 7.0 38.57±1.73 4.5 100.46±5.16 5.1 21.94±2.30 10.5 41.84±3.78 9.0 107.54±8.71 8.1 PC 12.10±0.32 2.6 24.50±1.18 4.8 94.50±0.97 1.0 13.36±0.45 3.4 32.45±1.01 3.1 103.79±1.57 1.5 AT-Ⅲ 14.10±0.88 6.2 23.90±0.88 3.7 110.40±1.07 1.0 15.04±0.98 6.5 31.09±1.62 5.2 111.17±2.03 1.8 vWF:Ag 28.71±0.90 3.1 60.17±1.63 2.7 106.47±2.34 2.2 30.33±1.13 3.6 59.95±1.24 2.1 103.63±4.23 4.1 PS、PC、AT-Ⅲ、vWF : Ag:同表 1;CV:同表 2 表 4 各脂肪酸与其他生化指标的相关系数
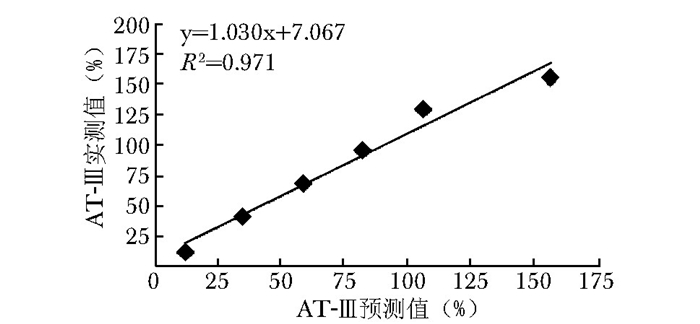
项目 r 偏差(%) 凝血因子 FⅡ 0.993 -8.5 FⅤ 0.983 15.5 FⅦ 0.998 3.7 FⅧ 0.975 3.9 FⅨ 0.993 -12.6 FⅩ 0.998 -11.7 FⅪ 0.989 7.7 FⅫ 0.990 7.4 易栓症筛查三项 PS 0.975 3.5 PC 0.993 3.3 AT-Ⅲ 0.983 15.2 vWF:Ag 0.975 9.3 PS、PC、AT-Ⅲ、vWF : Ag:同表 1 -
[1] National Committee for Clinical Laboratory Standards. Evaluation of precision performance of quantitative measurement methods; Approved guideline. EP5-A2[S]. Wayne, PA: NCCLS, 2004.
[2] National Committee for Clinical Laboratory Standards. Method comparison and bias estimation using patient samples; Approved guideline. EP9-A2[S]. Wayne, PA: NCCLS, 2002.
[3] 寿玮龄, 崔巍.恶性肿瘤出凝血异常机制[J].协和医学杂志, 2012, 4:482-486. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xhyx201204026 [4] Appert-Flory A, Fischer F, Jambou D, et al. Evaluation and performance characteristics of the automated coagulation analyzer ACL TOP[J]. Thromb Res, 2007, 120:733-743. DOI: 10.1016/j.thromres.2006.12.002
[5] 王海, 王成彬, 齐晓伟, 等.凝血功能分析的系统差异性对临床决策的影响探讨[J].中华临床医师杂志, 2012, 6:4371-4374. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhlcyszz201215047 [6] Bai B, Christie DJ, Gorman RT, et al. Comparison of optical and mechanical clot detection for routine coagulation testing in a large volume clinical laboratory[J]. Blood Coagul Fibrinolysis, 2008, 19:569-576. DOI: 10.1097/MBC.0b013e3283070872
[7] Dentali F, Sironi AP, Ageno W, et al. ABO blood group and vascular disease:an update[J]. Semin Thromb Hemost, 2014:49-59. http://www.ncbi.nlm.nih.gov/pubmed/24381150

 作者投稿
作者投稿 专家审稿
专家审稿 编辑办公
编辑办公 邮件订阅
邮件订阅 RSS
RSS

 下载:
下载: