Public's Knowledge and Experiences Towards Pediatric Clinical Trials: Methodological Characteristics Analysis
-
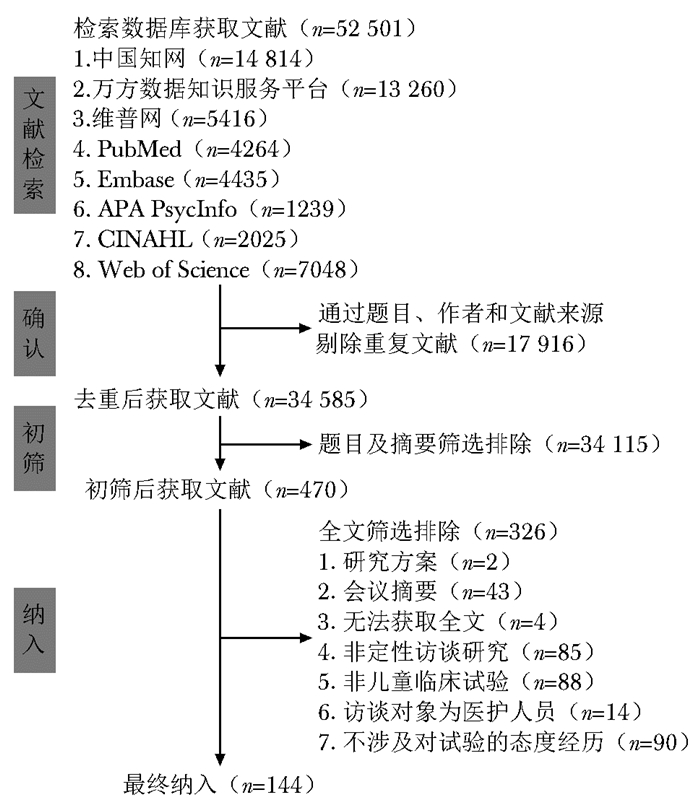
摘要:目的 分析儿童临床试验民众认知度及参与度定性访谈研究的方法学特征, 为我国开展儿童临床试验相关研究提供方法学参考。方法 计算机检索中国知网、万方数据知识服务平台、维普网、PubMed、Embase、APA PsycInfo、CINAHL和Web of Science数据库, 获取自建库至2021年9月21日发表的所有采用定性访谈研究的儿童临床试验民众认知度与参与度相关文献。8名研究人员随机分为4组, 2人一组经培训后使用Note Express(v 3.2)软件对文献进行预筛选和预提取, 经一致性检验合格后再独立进行文献筛选、资料提取和方法学特征分析。对于重复纳入、无法判断及观点不一致的文献均下载全文, 小组内筛选和记录排除原因, 如有分歧则经组内讨论后由团队负责人决定。采用CASP定性研究评价工具进行质量评价, 对提取资料进行归纳总结, 采用Microsoft Excel 2020软件进行数据整理。结果 共纳入英文文献144篇, 涉及137项研究, 其中80.3%(110/137)的研究无或有轻微质量担忧; 发表于2015—2021年的研究比例较高(54.7%, 75/137);共23个国家发表了相关研究, 其中以美国(37.2%, 51/137)和英国(26.3%, 36/137)最多; 75.9%(104/137)的研究仅基于真实儿童临床试验; 研究涉及最多的疾病类型为传染性疾病(28.9%, 35/121)和肿瘤(22.3%, 27/121);干预措施频次最高的是药物(38.6%, 39/101)和疫苗(14.9%, 15/101);招募方式多是医护人员在医院或诊所从临床试验受试者中招募访谈对象(43.0%, 49/114);访谈对象主要是儿童的父母(79.6%, 109/137);补偿方式多为代金券(46.7%, 21/45);访谈内容多是父母对儿童临床试验的认知及态度(27.5%, 78/284);多数研究采用半结构化访谈法(75.2%, 103/137)和面对面访谈形式(88.3%, 121/137);研究主要采用主题分析法(47.2%, 51/108)和NVivo软件(77.6%, 52/67)进行数据分析, 并运用多人独立分析法(46.4%, 52/112)和返回原始内容验证(77.8%, 21/27)以确保数据可靠性及可信度。结论 针对患儿及其父母的半结构化访谈结合主题分析法是目前儿童临床试验认知度与参与度定性访谈研究的主要方法。疾病类型以及干预措施是该类研究设计和实施重点关注的临床特征。建议我国在进行访谈内容设计和确定招募策略时需特别关注依从性差或疗效不佳患儿及其父母的观点和体验, 分析潜在受试患儿及其父母的认知、态度和经历, 为后续优化试验方案和提升试验质量提供参考。Abstract:Objective This study aims to analyze the methodological characteristics of qualitative interview studies investigating the public's knowledge and experiences regarding pediatric clinical trials and to provide reference for the design and implementation of similar research in China.Methods We conducted a comprehensive search in CNKI, WanFang Data, Vip Network, PubMed, Embase, APA PsycInfo, CINAHL, and Web of Science databases. We identified all published qualitative interview studies that explored the public's knowledge and experiences related to children's participation in clinical trials up to September 21, 2021. Eight researchers were divided into four groups, with two trained researchers in each group utilizing Note Express(v 3.2) software for pre-screening and data extraction. Literature screening, data extraction, and analysis were performed independently after consistency checks. Disagreements were resolved through team leader decisions after group discussion. The CASP Qualitative Checklist Tool was used to assess study quality, and the extracted data was summarized to develop a classification framework.Results A total of 144 English literature (137 studies) were included. The majority of the studies (80.3%, 110/137) exhibited minimal or no quality concerns. Most studies (54.7%, 75/137) were published from 2015 to 2021. Relevant studies were published in 23 countries, with the United States (37.2%, 51/137) and the United Kingdom (26.3%, 36/137) contributing the most. There were 75.9%(104/137) of studies focusing on actual pediatric clinical trials. The main diseases covered were infectious diseases (28.9%, 35/121) and cancer (22.3%, 27/121). Interventions in clinical trials predominantly involved drug therapy (38.6%, 39/101) and vaccines (14.9%, 15/101). Interviews were conducted by medical staff in hospitals or clinics, with participants mainly recruited from clinical trial subjects (43.0%, 49/114). Most interviewees were parents (79.6%, 109/137). Compensation was often provided in the form of vouchers (46.7%, 21/45). The content of included studies largely centered on parents' perspectives and perceptions of pediatric clinical trial knowledge and processes (27.5%, 78/284). Interviews were mainly performed as semi-structured interviews (75.2%, 103/137) and face-to-face interviews (88.3%, 121/137). Thematic analysis (47.2%, 51/108) and NVivo software (77.6%, 52/67) were commonly used for data analysis. Independent multi-person analysis (46.4%, 52/112) and content verification (77.8%, 21/27) were employed to ensure reliability and credibility.Conclusions Semi-structured in-depth interviews with parents and children using thematic analysis are a prominent research approach. Qualitative interview studies emphasize the clinical characteristics of diseases, including disease types and interventions. Specific attention should be given to perspectives and experiences of children and parents with poor adherence or clinical outcomes. Analyzing potential participants' knowledge, attitudes, and experiences can provide references for improving future trial design and quality.
-
Keywords:
- pediatric /
- clinical trial /
- attitude /
- knowledge /
- interview
-
宫颈癌是最常见的妇科恶性肿瘤,也是目前唯一有望被预防和消除的癌症。大量研究证实,持续感染高危型人乳头瘤病毒(high-risk human papillomavirus,HR-HPV)是导致宫颈癌发生的主要原因[1-2],因此探究如何预防HR-HPV感染具有重要意义。人乳头瘤病毒(human papilloma virus, HPV)主要通过性传播,性传播病原体除HPV外还包括淋病奈瑟球菌、沙眼衣原体(chlamydia trachomatis,CT)、生殖支原体(mycoplasma genitalium,MG)、单纯疱疹病毒(herpes simplex virus,HSV)等[3]。有研究表明[4-6],其他性传播病原体感染后可增加HPV的感染风险。本研究采用病例对照研究方法,探讨HR-HPV感染的危险因素,以期为宫颈癌预防提供参考依据。
1. 资料与方法
1.1 研究对象
以2020年10月—2021年1月就诊于首都医科大学附属北京妇产医院妇科门诊并自愿接受本调查研究的女性患者为研究对象,其中HR-HPV感染者作为感染组,HR-HPV阴性者作为对照组。
纳入标准:(1)育龄期女性;(2)有性生活史者。排除标准:(1)妊娠期、哺乳期或月经期女性;(2)绝经期女性;(3)72 h内有性生活或行阴道上药、阴道冲洗者;(4)因其他疾病长期应用激素、抗生素或免疫抑制剂者;(5)有全子宫切除手术史者;(6)因HR-HPV感染经阴道镜检查与病理活检确诊为宫颈癌者。
本研究经首都医科大学附属北京妇产医院伦理委员会审批通过(审批号:2021-KY-067-01),所有研究对象均签署知情同意书。
1.2 方法
1.2.1 HR-HPV分型及CT/MG检测
HR-HPV分型检测取样器为美国豪洛捷公司(Hologic Inc)指定宁波华莱斯医疗器械有限公司生产的一次性使用子宫颈细胞刷。通过刷取宫颈表面及宫颈管内样本,将样本转移至保存液中。将保存液瓶放入全自动HPV检测机,使用美国豪洛捷公司的Aptima HPV16/18/45 Genotype(AHPV GT)试剂盒(批号:295307DH)通过杂交捕获2代法检测其mRNA以确定HPV基因亚型。该方法共可检测16、18、31、33、35、39、45、51、52、56、58、59、66、68共14种HR-HPV亚型。
CT/MG检测是采用一次性无菌拭子分别取阴道后穹窿及宫颈管分泌物,使用上海仁度生物科技有限公司生产的CT/MG核酸检测试剂盒(批号:20201001)进行CT/MG核酸提取并应用实时荧光核酸恒温扩增检测技术进行检测。
1.2.2 调查工具
《HR-HPV感染相关危险因素调查表》为自行设计的问卷调查表,部分内容参考相关问卷[7-8],其内容包括研究对象的基本信息(年龄、受教育程度、职业、经济状况、烟酒嗜好)、卫生习惯(清洗外阴的方式、是否使用护垫、是否有阴道冲洗的习惯)、性生活情况、月经及婚育情况、既往阴道炎病史、阴道分泌物情况、妇科检查时外阴、宫颈口的情况。相较于国内其他学者设计的问卷调查表[7-8],本调查表更侧重于了解阴道微环境的情况及可能引起阴道微环境异常的卫生习惯、性生活情况、月经及生育情况。
1.2.3 实施方法
由经过培训的专科医生指导患者客观填写《HR-HPV感染相关危险因素调查表》。问卷中个人基本信息部分如年龄、受教育程度、职业、月收入情况均有相应选项供患者选择填写;从不吸烟者选择“否”,其余情况选择“是”;一周饮酒3次以上者选择“是”,其余情况选择“否”;清洁外阴方式、婚姻状况、初次性生活年龄、性伴侣数、性取向、性生活方式、避孕方式、生育次数、流产次数、是否经常使用护垫均有相应选项供患者选择填写;从不冲洗阴道者选择“否”,每个月经周期内冲洗阴道次数≤2次者选择“偶尔”,>2次者选择“经常”;月经周期频率为21~35 d且近1年月经周期之间的变化<7 d者选择“规律”,其余情况选择“不规律”;从未有过阴道炎病史者选择“无”,一年内阴道炎发作次数<4次者选择“有”,一年内阴道炎发作次数≥4次者选择“反复发作”;外阴或阴道烧灼痛情况选择按照疼痛评分,0分为“无”、1~4分为“轻度”、5~10分为“严重”;阴道分泌物量、阴道分泌物性状、分泌物清洁度、外阴红肿、宫颈口脓性分泌物、CT/MG感染情况由专科医生按照妇科检查及分泌物检查结果情况进行填写。
1.3 统计学处理
采用SPSS 22.0软件进行统计学分析。计量资料以均数±标准差表示,组间比较采用独立样本t检验;计数资料以例数和频数(百分比)表示,组间比较采用χ2检验或Fisher确切概率法;等级资料以例数和率表示,组间比较采用秩和检验。采用二分类Logistic回归法分析HR-HPV感染的危险因素。OR>1为危险因素,OR<1为保护因素。以P<0.05表示差异具有统计学意义。
2. 结果
2.1 两组一般情况
共纳入178例研究对象,其中感染组125例,对照组53例。感染组平均年龄(36.5±7.0)岁,对照组平均年龄(35.2±6.5)岁,两组差异无统计学意义(t=-1.103,P=0.272)。
2.2 HR-HPV感染相关因素的单因素分析
单因素组间比较结果显示,感染组无业或职业社会经济地位较低、用洗剂清洁外阴、冲洗阴道频率高、性取向为同性、生育次数多、有既往阴道炎病史、阴道分泌物量多、阴道分泌物性状异常和CT感染率均高于对照组(P均<0.05)。两组年龄、受教育程度、月收入、吸烟、酗酒、是否经常使用护垫、月经周期、婚姻状况、初次性生活年龄、性伴侣数、性生活方式、避孕方式、流产次数、分泌物清洁度、外阴红肿、宫颈口脓性分泌物、有无外阴或阴道烧灼痛、MG感染等指标比较差异无统计学意义(P均>0.05),见表 1。
表 1 HR-HPV感染相关因素的单因素分析[n(%)]因素 类别 感染组(n=125) 对照组(n=53) P值 因素 类别 感染组(n=125) 对照组(n=53) P值 年龄 20~24岁 2(1.6) 4(7.6) 0.762 性生活方式 单纯经阴道 110(88.0) 46(86.8) 0.823 25~29岁 19(15.2) 7(13.2) 方式多样 15(12.0) 7(13.2) 30~34岁 32(25.6) 10(18.9) 避孕方式 规范用避孕套 66(52.8) 29(54.7) 0.473 35~39岁 37(29.6) 16(30.2) 口服避孕药 3(2.4) 0(0) 40~44岁 16(12.8) 13(24.5) 宫内节育器 11(8.8) 3(5.7) 45~49岁 19(15.2) 3(5.7) 多种方式 17(13.6) 12(22.6) 受教育程度 专科或以下 40(32.0) 13(24.5) 0.302 未避孕 28(22.4) 9(17.0) 本科 62(49.6) 28(52.8) 生育次数 0次 37(29.6) 24(45.3) 0.034 研究生或以上 23(18.4) 12(22.6) 1次 60(48.0) 22(41.5) 职业 无 11(8.8) 2(3.8) <0.001 2次 28(22.4) 7(13.2) 工人/农民 11(8.8) 2(3.8) 3次 0(0) 0(0) 医疗卫生业 7(5.6) 15(28.3) 流产次数 0次 50(40.0) 18(34.0) 0.626 文艺工作者 3(2.4) 4(7.6) 1次 36(28.8) 15(28.3) 金融/经济 8(6.4) 6(11.3) 2次 23(18.4) 18(34.0) 信息技术业 7(5.6) 1(1.9) 3次 16(12.8) 2(3.8) 公务员 6(4.8) 0(0) 既往阴道炎病史 无 31(24.8) 24(45.3) <0.001 其他 72(57.6) 23(43.4) 有 73(58.4) 28(52.8) 月收入 <5000元 29(23.2) 8(15.1) 0.653 反复发作 21(16.8) 1(1.9) 5000~10 000元 43(34.4) 23(43.4) 阴道分泌物量 少 59(47.2) 6(11.3) <0.001 >10 000元 53(42.4) 22(41.5) 中等 53(42.4) 45(84.9) 吸烟 否 114(91.2) 52(98.1) 0.112 多 13(10.4) 2(3.8) 是 11(8.8) 1(1.9) 阴道分泌物性状 白色稀薄 98(78.4) 7(13.2) <0.001 酗酒 否 119(95.2) 53(100) 0.181 黄色均质 21(16.8) 5(9.4) 是 6(4.8) 0(0) 泡沫样 2(1.6) 0(0) 清洁外阴方式 清水 105(84.0) 51(96.2) 0.023 豆腐渣样 1(0.8) 0(0) 洗剂 20(16.0) 2(3.8) 血性 2(1.6) 1(1.9) 冲洗阴道 否 82(65.6) 46(86.8) 0.005 正常 1(0.8) 40(75.5) 偶尔 39(31.2) 6(11.3) 分泌物清洁度 Ⅰ度 95(76.0) 44(83.0) 0.281 经常 4(3.2) 1(1.9) Ⅱ度 24(19.2) 8(15.1) 是否经常使用护垫 否 53(42.4) 17(32.1) 0.319 Ⅲ度 6(4.8) 1(1.9) 偶尔 68(54.4) 36(67.9) 外阴红肿 无 125(100.0) 53(100.0) 1.000 经常 4(3.2) 0(0) 轻度 0(0) 0(0) 月经是否规律 否 29(23.2) 6(11.3) 0.068 严重 0(0) 0(0) 是 96(76.8) 47(88.7) 宫颈口脓性分泌物 无 122(97.6) 53(100.0) 0.556 是否已婚 否 24(19.2) 12(22.6) 0.601 有 3(2.4) 0(0) 是 101(80.8) 41(77.4) 外阴或阴道烧灼痛 无 120(96.0) 51(96.2) 0.966 初次性生活年龄 <15岁 4(3.2) 3(5.7) 0.980 轻度 5(4.0) 1(1.9) 15~20岁 35(28.0) 13(24.5) 严重 0(0) 1(1.9) > 20岁 86(68.8) 37(69.8) CT 阴性 109(87.2) 2(98.1) 0.024 性伴侣数 1人 99(79.2) 40(75.5) 0.582 阳性 16(12.8) 1(1.9) 2人 26(20.8) 13(24.5) MG 阴性 123(98.4) 2(98.1) 0.892 性取向 同性 19(15.2) 2(3.8) 0.031 阳性 2(1.6) 1(1.9) 异性 106(84.8) 51(96.2) HR-HPV: 高危型人乳头瘤病毒;CT: 沙眼衣原体;MG: 生殖支原体 2.3 两组HR-HPV感染相关因素的Logistic回归分析
因无业者仅有11人,不适合作为独立分组进行多因素分析,且职业分组过多,故对职业分组进行合并(将工人/农民归为蓝领,医疗卫生业、文艺工作者、金融/经济业、信息技术业、公务员归为白领)。将用洗剂清洁外阴和习惯冲洗阴道者合并为有清洁习惯、将阴道分泌物量多和阴道分泌物性状异常合并为阴道分泌物异常,最终选取职业、性取向、清洁习惯、生育次数、既往阴道炎病史、阴道分泌物异常、CT阳性7个因素作为自变量,HR-HPV感染情况作为因变量进行二分类Logistic回归分析。结果显示,生育史(OR=5.106,95% CI:1.521~17.145, P=0.008)、既往阴道炎病史(OR=3.910,95% CI:1.167~13.099, P=0.027)、阴道分泌物异常(OR=758.313,95% CI:58.151~9888.714, P<0.001)是HR-HPV感染的危险因素。此外,清洁习惯(OR=2.004)、性取向为同性(OR=13.972)、CT阳性(OR=15.058)均显示出与HR-HPV感染具有较强的关联性,但由于对照组样本较少,并未得出有显著意义的结果,见表 2。
表 2 HR-HPV感染相关因素的Logistic回归分析变量 偏回归系数 标准误 Wald χ2值 P值 OR值 95% CI 下限 上限 职业(白领/蓝领或无业) 0.114 0.497 0.053 0.819 0.818 0.113 5.930 职业(其他/蓝领或无业) -0.428 0.423 1.027 0.311 0.476 0.081 2.796 清洁习惯(有/无) 0.348 0.308 1.274 0.259 2.004 0.599 6.704 性取向(同性/异性) 1.319 1.026 1.653 0.199 13.972 0.251 778.222 生育史(有/无) 0.815 0.309 6.961 0.008 5.106 1.521 17.145 既往阴道炎病史(有/无) 0.682 0.309 4.884 0.027 3.910 1.167 13.099 阴道分泌物异常(是/否) 3.316 0.655 25.613 <0.001 758.313 58.151 9888.714 CT(阳性/阴性) 1.356 0.733 3.419 0.064 15.058 0.850 266.756 HR-HPV、CT: 同表 1 3. 讨论
研究证明,HPV感染是多种因素协同作用的结果,已被报道的HPV感染危险因素包括初次性生活年龄、妊娠及流产次数、性伴侣数、避孕方式、泌尿生殖道感染等[7-8]。研究纳入的人群及对照人群所属地区及种族不同,得出的结论也不同。
虽然HPV感染是宫颈病变发生、发展的必要因素,但感染HPV并不等同于宫颈病变。魏丽惠[9]研究表明,从最初感染HPV到最终发生宫颈癌变需要一定的条件,约80%的女性一生中会感染HPV,其中70%所感染的HPV可于1年内自行清除,约90%所感染的HPV可于2年内自行清除。如病毒仍持续作用于宫颈,有约7%的HPV感染者在5~10年后进展为宫颈癌[10]。本研究结果显示,HR-HPV感染的3个危险因素可能是导致HR-HPV持续感染、阻碍其自行清除的关键原因。分娩不可避免地使生殖道黏膜经历损伤和再修复的过程,该过程中黏膜自身保护屏障的短时或长时缺失,可能为HPV的入侵提供了可乘之机。如果阴道微生态环境处于失调状态,即本文中所提到的阴道炎病史,通常表现为白带增多和性状异常,会使机体对HPV的清除能力下降,从而使宫颈、阴道、外阴、肛周长期处于HPV持续感染和攻击的环境中[11-12],最终导致宫颈病变。而HR-HPV感染又会导致阴道微生态环境的异常[12-13],二者互为因果,相互作用。黄晓澜等[14]认为,HR-HPV感染后,如患有阴道炎,可能会导致宫颈癌的发生。阴道炎除能损伤阴道黏膜、破坏阴道微生态环境、抑制免疫功能外,还与HPV感染、宫颈癌前病变和宫颈癌的发生发展密切相关[15-16]。
此外,清洁习惯、性取向为同性、CT阳性均显示出与HR-HPV感染具有较强的关联性。不正确的清洁习惯往往是破坏阴道微生态环境的罪魁祸首。研究证实[13],过分追求外阴、阴道“清洁”的人群,阴道炎的发病率及感染HPV的概率明显升高。而性取向为同性者,其性生活中使用的工具或器械会增加阴道黏膜损伤的几率,从而为HPV的感染创造了可乘之机。随着阴道微生态环境研究的进展,越来越多的研究表明,一种性传播病原体是另一种性传播病原体感染的高危因素。Naldini等[17]研究表明HPV和CT互为感染的危险因素。HR-HPV感染或CT感染的女性需要进行共感染的筛查从而对可能出现的严重生殖健康结果(例如宫颈癌和不孕症)进行预防性干预。Koster等[18]发现HPV和CT共感染能引发宿主细胞重编程,二者从相反的方向影响细胞和基因组稳定性,从而促进肿瘤的形成。本研究未得出CT感染是HR-HPV感染的危险因素相关结论,可能与选取的研究对象有关:排除了因HR-HPV感染经阴道镜检查及病理活检确诊为宫颈癌的患者,仅研究无宫颈病变或宫颈低级别及高级别鳞状上皮内病变患者。本研究也未得出清洁习惯、性取向为同性是HR-HPV感染的危险因素相关结论,这可能与样本量相对较少有关,导致在单因素分析中两个因素在两组间有差异,但在多因素分析中OR值置信区间过宽,未能得出有统计学差异的结论。我们将会在后续的研究中,进一步增加样本量,并增加HR-HPV感染与阴道分泌物pH值等相关因素研究[19-20],从而进一步明确HR-HPV感染的相关危险因素。
综上所述,HR-HPV感染与多种因素相关,有生育史、既往阴道炎病史、阴道分泌物异常是HR-HPV感染的危险因素。建议重视阴道炎的治疗,关注阴道分泌物变化,如有异常,及时就诊。在有条件的情况下,行宫颈HR-HPV筛查时加入阴道微生态的相关检查,如有生殖道炎性疾病,应及时治疗,从多方面预防HR-HPV的感染及降低HR-HPV持续感染的风险。
志谢: 感谢北京中医药大学中医学院学生丁梦瑶、郭亚坤、姜亚彤、李佳颖、刘东华、刘思岐、盛竹君、杨馥伊、杨诗宇、要丹柠、曾斌、张佳坤、张剑雨、赵书晗、周梦情在资料提取工作中提供的帮助。作者贡献:费宇彤负责研究构思;费宇彤、于明坤负责研究设计;任毅铭、李睿、唐思、吉广荷、秦泽琳、赵乐滢、佟佳曦、陈钰璇、高佳琪负责数据收集和处理;任毅铭、李睿、唐思、吉广荷、秦泽琳负责数据分析整理;任毅铭负责论文撰写;费宇彤、夏如玉、荣红国负责论文修订及审核。利益冲突:所有作者均声明不存在利益冲突 -
表 1 纳入研究的基本特征(n=137)
指标 研究数量(n) 占比(%) 指标 研究数量(n) 占比(%) 疾病类型(n=121) 招募地点(n=114) 传染性疾病1) 35 28.9 医院/诊所 23 20.2 肿瘤2) 27 22.3 社区/乡村 9 7.9 呼吸系统1) 20 16.5 学校 6 5.3 血液病2, 3) 20 16.5 其他8) 5 4.4 神经系统1, 2) 20 16.5 招募人员(n=114) 内分泌系统 12 9.9 医护人员 20 17.5 遗传病3) 10 8.3 社会工作者 3 2.6 早产儿疾病 9 7.4 研究团队 5 4.4 消化系统 6 5.0 研究助理 3 2.6 精神类疾病 6 5.0 研究负责人 3 2.6 自身免疫病4) 5 4.1 补偿方式(n=45) 过敏性疾病5) 4 3.3 代金券 21 46.7 皮肤病1, 4) 4 3.3 现金 17 37.8 肾脏疾病 4 3.3 交通费补贴 11 24.5 先天性心脏病 4 3.3 免费食品 7 15.6 其他6) 12 9.9 其他 4 8.9 干预措施(n=101) 访谈对象(n=137)9) 药物 39 38.6 父母 109(6065) 79.6 疫苗 15 14.9 儿童/青少年 52(2115) 38.0 预防/检查 11 10.9 祖父母/长老 4(13) 2.9 语言/行为/心理 9 8.9 社区领袖 4(57) 2.9 手术 9 8.9 兄弟姐妹 3(39) 2.2 危重症治疗 5 5.0 老师/校长 3(74) 2.2 干细胞移植 4 4.0 社区居民 2(25) 1.5 放疗 3 3.0 成年患者 1(17) 0.7 饮食干预 2 2.0 其他10) 2(5) 1.5 其他 12 11.9 访谈人员(n=137)11) 招募方式(n=114) 研究人员 43(58.1) 31.4 通过试验7) 49 43.0 文章作者 40(45.0) 29.2 广告宣传 33 28.9 研究助理 12(83.3) 8.8 机构/组织 21 18.4 心理学家 10(60.0) 7.3 邮寄信件 12 10.5 医护人员 4(75.0) 2.9 面对面介绍 12 10.5 社会学家 3(66.7) 2.2 电话/短信 12 10.5 社区工作者 3(33.3) 2.2 电子邮件 10 8.8 未提及 36 26.3 活动/会议/宣讲 7 6.1 调查问卷 6 5.3 口口相传 4 3.5 1)8项合并呼吸系统疾病,4项合并神经系统疾病,1项合并皮肤病;2)18项合并血液病,7项合并神经系统疾病;3)此处遗传病不涉及多基因及线粒体异常,2项合并血液病, 1项合并神经系统疾病;4)1项合并皮肤病;5)2项合并皮肤病,不包含过敏性哮喘;6)包括眼科、口腔、肥胖、早孕、性健康、麻醉、创伤性损伤;7)指研究者或医护人员通过既往或现行的临床试验受试者群体寻找访谈对象;8)2项在研究中心,1项在患者注册处,1项在教堂,1项在托儿所;9)括号内是该类型访谈对象总人数;10)同伴教育者3人,权益倡导者2人;11)括号内是经过训练或经验丰富的访谈人员占比 表 2 访谈内容(n=137)
访谈对象 试验知识与过程/伦理问题/疾病类型/干预措施 认知理解 观点态度 经历体验 促进/阻碍因素 改善建议 父母 21(12/0/7/2) 57(30/4/11/12) 42(16/0/21/5) 55(25/1/18/11) 11(7/0/2/2) 儿童/青少年 9(5/0/1/3) 16(12/2/1/1) 24(9/0/12/3) 29(12/0/12/5) 1(1/0/0/0) 兄弟姐妹 2(0/0/2/0) 0 2(0/0/2/0) 0 0 教师/校长 0 1(0/1/0/0) 0 3(0/0/1/2) 0 社区居民 0 4(1/0/0/3) 0 4(3/0/0/1) 0 社会组织 0 1(0/1/0/0) 0 0 2(2/0/0/0) 总计 22 64 39 56 7 注:试验知识及过程包括宣传招募、决策过程、家庭共同决策、知情同意、非事先知情同意、随机程序、利他主义、非治疗受益、试验知识、试验过程、依从性、感谢信、试验结果;伦理问题包括疫苗伦理、艾滋病伦理、干细胞伦理、基因捐赠;疾病类型包括癌症、糖尿病、炎性肠病、早产儿、婴儿癫痫、先天性心脏病、肌营养不良症、Down综合征、脆性X染色体综合征、抑郁症、生殖健康、营养不良、儿童肥胖;干预措施包括急救护理、干细胞移植、阴道杀菌剂、膳食与康复、病毒检测、侵入性操作、暴露前预防、艾滋病疫苗、流感疫苗、登革热疫苗、结核疫苗、脊灰疫苗、疟疾疫苗、疟疾预防、疟疾治疗、基因治疗、膳食与康复、精准医疗;社会组织包括社区护工、艾滋病咨询委员会和非政府组织、妇女组织等 表 3 纳入研究的方法学特征(n=137)
方法学特征 研究数量 占比(%) 方法学特征 研究数量 占比(%) 访谈方法(n=137) 分析人员合作模式(n=88) 半结构化访谈法 103 75.2 两人及以上研究者独立分析,讨论解决分歧 52 59.1 无结构式访谈 12 8.8 两人分析,与团队讨论或第三个人检查 8 9.1 结构化访谈法 2 1.5 团队一起分析,讨论达成共识 4 4.5 焦点小组访谈法 40 29.2 一人分析,团队讨论或其他人检查监督 18 20.5 线上非同步焦点小组访谈法 3 2.2 一人主导分析,其他人协助 6 6.8 资料收集方法(n=137) 定性分析软件(n=67) 面对面访谈1) 121 88.3 NVivo 52 77.6 电话访谈 43 31.4 ATLAS.ti 12 17.9 录音并转录成文本 100 73.0 MaxQDA 2 3.0 田野笔记 27 19.7 NUD*IST 1 1.5 表情和肢体动作等非语言内容 19 13.9 可靠性验证方法(n=112) 分析方法(n=108) 多人独立分析 52 46.4 主题分析法 51 47.2 抽取部分内容分析核对 5 4.5 归纳分析法 14 13.0 三角验证5) 14 12.5 归纳演绎法 4 3.7 与备忘录和田野笔记比对 10 8.9 框架分析法 13 12.0 审查追踪6) 7 6.3 定性分析法 12 11.1 可信性验证方法(n=27) 内容分析法 9 8.3 返回原始内容验证 21 77.8 持续比较法 9 8.3 第三方讨论7) 4 14.8 描述分析法 8 7.4 检索关联外部文献 3 11.1 其他分析方法 5 4.6 分析步骤(n=124) 基本符合主题分析步骤2) 61 49.2 基本符合框架分析步骤3) 58 46.8 采用框架或编码手册4) 45 36.3 使用归纳步骤 100 80.6 使用归纳演绎步骤 23 18.5 使用演绎步骤 1 0.8 1)面对面和电话形式均包括的研究为27项;2)主题分析步骤:熟悉数据,初始编码,寻找主题,回顾主题,定义主题,形成报告[4];3)框架分析步骤:熟悉数据,识别框架,标引数据,形成图表,进行解释[5];4)手册形成步骤:初步熟悉,预编码或从访谈提纲中,形成初步框架,据此进行后续编码并不断完善,团队讨论并形成最终编码手册;5)即不同分析人员、不同分析方法或不同数据来源;6)记录整个研究过程以便同行审议,包括原始数据、数据简化分析和数据重组综合[6];7)医护人员、患儿及家属代表、当地研究者、当地卫生工作者等 表 4 民众对儿童临床试验认知度及参与度的访谈研究设计
研究步骤 内容 研究背景 临床试验的基本信息(设计类型、疾病类型和干预措施等) 访谈对象 现有儿童受试者、潜在儿童受试者、父母或监护人、其他家属、老师、校长和社区居民访谈对象的基本特征(年龄、性别、民族、受教育程度和宗教信仰)患儿是否与父母一起接受访谈 访谈内容 认知理解、观点态度、经历体验、促进/阻碍因素、改善建议可涉及试验知识及过程、伦理问题、疾病类型和干预措施等方面 招募方法 通过现有试验受试者人群、广告/媒体宣传、机构组织协助面对面介绍、电子邮件、电话和短信等方式 访谈人员 医护人员、临床科研人员、方法学家、社会学家、心理学家经过培训或经验丰富 访谈方法 “一对一”半结构化深度访谈或焦点小组访谈面对面、电话或线上访谈 分析方法 主题分析法或框架分析法;如有必要,采用扎根理论等采用定性分析软件,规范化分析步骤 结果验证 多人独自分析、三角验证或审查追踪验证可靠性返回原始资料、检索其他文献或与第三方讨论验证可信度 -
[1] 严恺, 孔艳婷, 葛萌萌, 等. 1998—2015年全球儿童临床试验注册趋势分析[C]. 浙江省医学会儿科学分会, 江苏省医学会儿科学分会, 上海市医学会儿科学分会. 第十三届江浙沪儿科学术会议暨2016年浙江省医学会儿科学学术年会论文汇编, 2016: 686-687. [2] Nabulsi M, Khalil Y, Makhoul J. Parental attitudes towards and perceptions of their children's participation in clinical research: a developing-country perspective[J]. J Med Ethics, 2011, 37: 420-423. DOI: 10.1136/jme.2010.035899
[3] Wright EB, Holcombe C, Salmon P. Doctor's communica-tion of trust, care, and respect in breast cancer: qualitative study[J]. BMJ, 2004, 328: 864. DOI: 10.1136/bmj.38046.771308.7C
[4] Braun V, Clarke V. Using thematic analysis in psychology[J]. Qual Res Psychol, 2006, 3: 77-101. DOI: 10.1191/1478088706qp063oa
[5] Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research[J]. Qualitative Report, 2021, 26: 2061-2076.
[6] Wolf ZR. Exploring the audit trail for qualitative investiga-tions[J]. Nurse Educ, 2003, 28: 175-178. DOI: 10.1097/00006223-200307000-00008
[7] 潘薇薇, 倪韶青, 李春梅, 等. 8~18岁儿童对临床研究认知的调查[J]. 中华儿科杂志, 2019, 57: 876-881. [8] 潘薇薇, 倪韶青, 李春梅, 等. 对公众儿童临床研究认知的调查[J]. 中华医院管理杂志, 2017, 33: 75-78. [9] 李莉霞, 王晓芸, 李方, 等. 家长对儿童药物临床试验认知与态度调查[J]. 儿科药学杂志, 2019, 25: 43-47. https://www.cnki.com.cn/Article/CJFDTOTAL-EKYX201903016.htm [10] 李莉霞, 陆晓彤. 监护人参与儿童药物临床试验的经历与知情同意认知度调研[J]. 儿科药学杂志, 2021, 27: 39-42. https://www.cnki.com.cn/Article/CJFDTOTAL-EKYX202108012.htm [11] 陈荷娣. 家长对儿童药物临床试验认知状况的调查分析[J]. 上海护理, 2015, 15: 27-29. https://www.cnki.com.cn/Article/CJFDTOTAL-SHHL201501011.htm [12] 张清华, 冉素娟, 李洁, 等. 患儿法定监护人对药物临床试验的认识、态度及关注点研究[J]. 中国新药与临床杂志, 2016, 35: 555-560. https://www.cnki.com.cn/Article/CJFDTOTAL-XYYL201608007.htm [13] 张清华. 重庆地区医患双方对儿童临床试验的认识、态度及影响因素研究[D]. 重庆: 重庆医科大学, 2016. -
期刊类型引用(1)
1. 刘敏,杨金苹,赵金颜,乔建红. 老年共病患者自我感知老化、抑郁情绪与生活质量的相关性研究. 心理月刊. 2024(21): 58-60 .  百度学术
百度学术
其他类型引用(0)

 作者投稿
作者投稿 专家审稿
专家审稿 编辑办公
编辑办公 邮件订阅
邮件订阅 RSS
RSS

 下载:
下载: