-
摘要: 异柠檬酸脱氢酶(isocitrate dehydrogenase,IDH)作为三羧酸循环的关键酶,在细胞能量代谢中发挥重要作用。IDH基因突变可引起该酶的活性发生改变,导致大量肿瘤代谢物2-羟基戊二酸蓄积,从而引起严重的表观遗传调控紊乱和基因表达失调,促进肿瘤发生。近年来研究表明,IDH1和IDH2突变与胶质瘤、急性髓系白血病、肝内胆管癌等多种肿瘤的发生发展及其临床治疗具有密切关系。将IDH基因突变作为检测胶质瘤、急性髓系白血病、肝内胆管癌等肿瘤发生的分子标志物,以及靶向药物的开发位点具有重要意义。本文就IDH突变机制及其与多种肿瘤发生发展的关系,以及IDH突变治疗在基础研究和药物临床研究中的进展作一综述。Abstract: Isocitrate dehydrogenase (IDH), a key enzyme in the tricarboxylic acid cycle, plays an important role in cellular energy metabolism. When the IDH gene is mutated, the enzyme activity is altered, resulting in an accumulation of a large amount of the tumor metabolite 2-hydroxyglutaric acid (2-HG), causing severe epigenetic deregulation and dysregulation of gene expression, and promoting tumorigenesis. Recent studies have shown that IDH1 and IDH2 mutations are closely related to the occurrence and development of a variety of tumors such as glioma, acute myeloid leukemia(AML) and intrahepatic cholangiocarcinoma(iCCA) as well as their clinical treatment. It is of great significance to use IDH gene mutations as molecular indicators for detecting glioma, AML and iCCA tumorigenesis, and developing targeted drugs. This review is focused on the mechanism of IDH mutation, the relationship between mutant IDH and the development of multiple tumors, and the progress of treatment of IDH mutation in basic research and drug clinical trials.
-
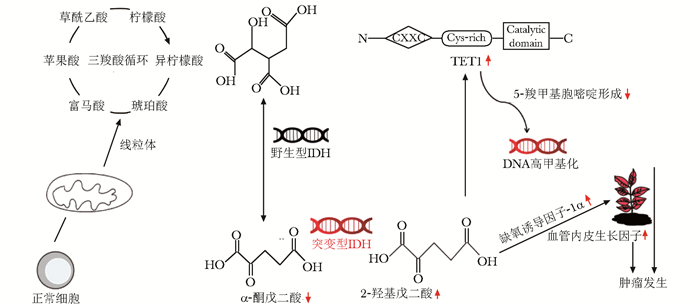
异柠檬酸脱氢酶(isocitrate dehydrogenase,IDH)1和IDH 2在葡萄糖感知通路、谷氨酰胺代谢、脂肪生成及细胞活性氧调节等生命活动中发挥重要作用[1],可催化异柠檬酸生成α-酮戊二酸(α-Ketoglutaric acid,α-KG)、还原型烟酰胺腺嘌呤二核苷酸磷酸(reduced nicotinamide adenine dinucleotide phosphate oxidase, NADPH)和还原型烟酰胺腺嘌呤二核苷酸(reduced nicotinamide adenine dinucleotide, NADH),而NAD(P)H可维持谷胱甘肽(glutathione,GSH)和硫氧还蛋白(thioredoxin,Trx)处于还原状态以抵御细胞氧化反应和线粒体氧化损伤[2-3]。野生型IDH形成同源二聚体发挥功能,而突变型IDH无法与野生型IDH形成二聚体,催化底物生成α-KG,进而产生2-羟基戊二酸(2-hydroxyglutaric acid,2-HG)这一致癌性代谢产物参与肿瘤发生发展[4]。IDH基因突变较常出现在胶质瘤、急性髓系白血病(acute myeloid leukemia,AML)和肝内胆管癌(intrahepatic cholangiocarcinoma,iCCA)中[5-7]。随着精准医疗及分子病理学等新兴学科的发展,以IDH作为分子靶点的靶向治疗及预后预测具有重要的科研意义和临床应用价值。本文就IDH基因突变机制及其在肿瘤治疗中的研究进展作一综述。
1. IDH基因突变及其致癌机制
IDH是三羧酸循环的关键酶,可催化异柠檬酸氧化脱羧生成α-KG及CO2,并将氧化型烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide, NAD+)/氧化型烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate, NADP+)(NAD+/NADP+)还原为NADH/NADPH,为细胞能量代谢和生物合成提供前体物质[8]。IDH包括IDH1、IDH2和IDH3,其中IDH1/IDH2基因突变可引起α-KG减少和2-HG生成[9]。组蛋白去甲基化酶和DNA去甲基化酶10-11易位(ten-eleven translocation,TET) 蛋白是依赖于α-KG的双加氧酶,参与血管生成、缺氧应激、细胞的成熟分化等生理病理过程[10]。2-HG水平升高可抑制TET甲基胞嘧啶双加氧酶1活性,导致5-甲基胞嘧啶(5-methylcytosine, 5mC) 无法转变为5-羟甲基胞嘧啶(5-hydroxymethylcytosine,5hmC),使DNA与组蛋白处于高甲基化状态;α-KG减少可促进缺氧诱导因子-1α(hypoxia inducible factor-1α,HIF-1α)积聚并激活相关信号通路,引起下游血管内皮生长因子(vascular endothelial growth factor,VEGF)、磷酸甘油酸激酶(Phosphoglycerate kinase, PGK)等肿瘤相关基因高表达,共同促进肿瘤的发生与发展(图 1)[11-12]。
2. IDH基因突变与肿瘤治疗的相关性
目前,越来越多的研究表明IDH基因突变与多种肿瘤发生、发展和预后密切相关。胶质瘤是成人、儿童和青少年最常见的中枢神经系统肿瘤[13]。IDH1突变常见于低级别胶质瘤或继发性胶质母细胞瘤中,在原发性胶质母细胞瘤中较少见[14]。与IDH1野生型胶质瘤患者相比,IDH1突变的胶质瘤患者对放化疗的敏感性更高,预后更好,中位总生存期(median overall survival,mOS)可延长16个月[15]。约20%的AML患者存在IDH1/2突变,与其他肿瘤不同,AML患者中IDH2突变率高于IDH1[16],且IDH基因突变的亚型不同其预后也不同,IDH1突变患者预后较差,而IDH2突变患者预后较好[17]。iCCA是起源于胆管上皮的原发性肝内恶性肿瘤[18],近年来其发病率和死亡率均呈上升趋势[19]。约20%的iCCA患者存在IDH1突变,IDH1 R132C是iCCA中最常见的突变位点[20]。研究发现,IDH1/2突变的iCCA患者1、4和7年肿瘤复发率(分别为10.5%、45.3%和45.3%)显著低于IDH野生型iCCA患者(分别为41.7%、71.5%和81.3%)[21]。多项研究显示,与IDH野生型肿瘤相比,IDH突变型肿瘤通过下调NAD+挽救途径中的烟酸磷酸核糖转移酶(nicotic acid phosphoribosyltransferase,Naprt)活性以降低NAD+水平,增加IDH突变的iCCA患者对替莫唑胺(temozolomide,TMZ)和聚二磷酸腺苷核糖聚合酶抑制剂(poly adenosine diphosphate ribose polymerase inhibitor,PARPi)等多种药物的敏感性,从而改善患者预后[22-23]。
3. IDH基因突变相关肿瘤治疗进展
3.1 化疗
目前,IDH突变的胶质瘤患者首选TMZ+辅助性PCV(丙卡巴肼、洛莫司汀、长春新碱)化疗方案[24]。研究发现,在2种IDH1 R132H突变胶质瘤模型中,5-氮杂胞苷单药治疗可抑制肿瘤细胞生长及诱导细胞分化[25]。自然杀伤(natural killer,NK) 细胞通过激活自然杀伤细胞2族成员D(natural killer group 2 member D,NKG2D)受体识别肿瘤细胞上的配体,从而杀伤肿瘤细胞[26]。IDH突变胶质瘤中DNA呈高甲基化状态,可抑制NKG2D配体UL16BP1和UL16BP3的转录,使NK细胞无法杀伤肿瘤细胞,从而导致肿瘤发生[27]。去甲基化药物5-氮杂-2-脱氧胞苷作为DNA甲基转移酶(DNA methyltransferase,DNMT)1抑制剂,能诱导IDH突变胶质瘤DNA低甲基化,恢复NK细胞对肿瘤细胞的杀伤活性,抑制肿瘤生长。5-氮杂胞苷单药通过抑制DNMT活性,特异性抑制DNA和组蛋白高甲基化,与艾伏尼布作用机制相似。一项艾伏尼布和阿扎胞苷联合治疗23例IDH1突变AML患者的Ⅰ/Ⅱ期临床试验(NCT02677922)显示,总有效率为78.3%,完全缓解率达60.9%[28]。目前,IDH突变的iCCA患者首选吉西他滨+顺铂联合化疗方案,mOS和中位无进展生存期(median progression-free survival,mPFS)分别为11.7个月和8.0个月[29]。
3.2 免疫疗法
免疫疗法主要包括单克隆抗体疗法、疫苗疗法、嵌合抗原受体工程化T淋巴细胞疗法和检查点抑制剂疗法[30-31]。T淋巴细胞受体通过识别肿瘤细胞表面的人白细胞抗原(human leukocyte antigen,HLA)分子,诱导特异性T淋巴细胞应答,从而发挥抗肿瘤免疫反应[32]。一项IDH1肽疫苗治疗IDH1 R132H突变的Ⅲ~Ⅳ级胶质瘤Ⅰ期临床试验(NCT02454634)表明,其可诱导93.3%的患者发生免疫应答,82%的患者在2年内无疾病进展[33]。研究表明,与单独接种IDH1肽疫苗相比,疫苗接种与IDH抑制剂联合治疗,可显著提高IDH1 R132H突变胶质瘤小鼠的存活率[34]。目前关于程序性死亡[蛋白]配体1(programmed death-ligand 1,PD-L1)抑制剂与IDH1 R132H特异性疫苗联合治疗胶质瘤患者的Ⅰ期临床试验(NCT03893903) 正在进行中。Bunse等[35]发现,在小鼠胶质瘤模型中,程序性死亡[蛋白]-1(programmed death-1,PD-1) 抑制剂与IDH1抑制剂协同治疗可缩小小鼠肿瘤体积并延长生存时间。目前已有3项PD-1抑制剂治疗IDH突变胶质瘤的临床试验(NCT03557359、NCT03718767、NCT03925246)正在开展中,试验结果尚未公布。
3.3 靶向治疗
3.3.1 IDH抑制剂
IDH抑制剂通过作用于肿瘤细胞中的IDH突变位点,将2-HG浓度降为正常水平,从而诱导组蛋白和DNA去甲基化,抑制肿瘤生长。根据作用靶点不同,可将IDH抑制剂分为IDH1抑制剂、IDH2抑制剂和IDH1/2抑制剂3种。
IDH1抑制剂:艾伏尼布为口服、靶向、小分子针对IDH1突变的抑制剂。DiNardo等[36]对IDH1突变AML患者进行艾伏尼布单药治疗的Ⅰ期临床试验(NCT02074839)表明,在复发或难治性AML初治有效的125例患者中,完全缓解或完全缓解伴血液学部分恢复者占30.4%,完全缓解率为21.6%,总有效率为41.6%;7例未检测到残留的IDH1突变,安全性数据显示,至少3例发生了与治疗相关的不良事件(adverse event,AE)(≥3级),包括QT间期延长、IDH分化综合征、贫血、血小板减少和白细胞增多。Mellinghoff等[37]研究发现,500 mg/d剂量的艾伏尼布治疗IDH1突变晚期胶质瘤可获得良好的安全性,能够延长疾病控制时间,减缓无强化肿瘤的生长。Andronesi等[38]的Ⅰ期临床研究结果显示艾伏尼布治疗后1周,2-HG水平迅速下降70%(95% CI: 0.043~0.091,P=0.019)。一项评估艾伏尼布治疗IDH1突变实体瘤的安全性和耐受性Ⅰ期临床试验(NCT02073994)表明, 73例IDH1突变晚期胆管癌(cholangiocarcinoma,CCA)接受艾伏尼布(500 mg/d) 治疗,结果显示mPFS为3.8个月,mOS为13.8个月[39];安全性数据显示,多数AE为1~2级,乏力(42%)、恶心(34%)、腹泻(32%)、腹痛(27%)、食欲减退(27%)和呕吐(23%) 最为常见,其中46例出现与治疗有关的AE,其中4例出现AE(≥3级),包括疲乏、血磷降低和血碱性磷酸酶升高。另外,一项国际多中心Ⅲ期临床试验(NCT02989857)比较了艾伏尼布与安慰剂治疗不可切除或转移性IDH1突变CCA患者的结果表明,与安慰剂组相比,艾伏尼布组mPFS显著延长(1.4个月比2.7个月)[40];安全性数据显示,最常见的AE(≥3级)为腹水(7%),艾伏尼布组中有3例出现与治疗有关的AE(≥3级),包括高胆红素血症、黄疸、胸腔积液,两组均未出现与治疗相关的死亡病例。一项IDH1抑制剂IDH305治疗IDH1突变异种移植瘤的研究表明,2-HG浓度降低与疗效存在相关性,目前已开展相关临床试验[41]。IDH1抑制剂BAY1436032能够特异性抑制2-HG的生成和集落生长,并诱导携带泛IDH1多位点突变的AML细胞向髓系分化,目前已开展相关临床试验[42]。
IDH2抑制剂:伊那尼布为口服、可逆、选择性的针对IDH2突变的抑制剂,可促进AML成髓细胞分化,是首个IDH2抑制剂,其在体外与IDH2突变体R140Q、R172S和R172K结合能力高于野生型IDH2 40倍,而对IDH1突变体无抑制作用,于2017年被美国食品药品监督管理局(Food and Drug Administration,FDA)批准上市,用于治疗复发/难治性IDH2突变AML[43]。一项伊那尼布单药治疗IDH2突变AML患者的Ⅰ/Ⅱ期临床试验(NCT01915498)显示,患者的mOS为11.3个月[44];安全性数据显示,与治疗相关的AE包括高胆红素血症(31%)、恶心(23%)、血细胞减少(21%)、疲劳(18%)、食欲下降(18%)和皮疹(18%)。一项伊那尼布50~650 mg/d,每28天为一个周期治疗mIDH2血液病的Ⅰ/Ⅱ期临床试验(NCT01915498)发现,IDH2-R140或IDH2-R172突变AML患者的反应和存活率相似,患者的mOS为8.8个月[45],安全性数据显示,与治疗相关的AE(≥3级)包括高胆红素血症(10%)、血小板减少(7%)和IDH分化综合征(6%)。因此,新诊断IDH2突变AML老年患者可从伊那尼布靶向治疗中获益。
IDH1/2抑制剂:伏昔尼布为口服、高脑渗透性的第二代针对IDH1/2突变的双重抑制剂[46]。研究发现,在IDH突变胶质瘤的原位模型中,伏昔尼布可显著抑制肿瘤生长和2-HG水平[47]。由于突变型IDH1与突变型IDH2的异构体相互转换已成为AML获得性耐药的潜在机制,因此突变型IDH1和突变型IDH2的双重抑癌作用优于单-异构体选择性抑癌作用[9]。一项多中心、开放标签、Ⅰ期、剂量递增的伏昔尼布临床试验(NCT02481154) 共入组93例IDH1/2突变晚期实体瘤患者(包括52例胶质瘤),治疗方案为口服给药、每日1次,每28 d为1个周期,直至疾病进展或发生不可接受的毒性,结果显示,伏昔尼布在剂量<100 mg/d时表现出良好的安全性,在无强化胶质瘤患者中的mPFS为36.8个月,增强型胶质瘤患者的mPFS为3.6个月,无强化IDH1/2突变胶质瘤患者的耐受性良好[46],安全性数据显示,64例出现与治疗相关的AE,其中29例出现≥3级AE[癫痫(7.7%)最为常见],无治疗相关死亡病例。目前,已开展伏昔尼布50 mg/d与安慰剂治疗术后复发的Ⅱ级无强化IDH突变胶质瘤患者的临床试验(NCT04164901)。
3.3.2 其他抑制剂
多腺苷二磷酸核糖聚合酶[poly(ADP-ribose) polymerase,PARP] 抑制剂:其是由PARP、谷氨酰胺代谢和Bcl-2蛋白家族的化合物诱导合成为靶向药物[48]。Sulkowski等[11]报道IDH1/2突变诱导同源重组(homologous recombination,HR)缺陷,使肿瘤细胞对PARP抑制剂高度敏感。临床前研究表明,药物抑制PARP活性可显著提高其他治疗方法的疗效,包括细胞毒性药物和表观遗传化疗、小分子抑制剂和抗体药物结合物[49]。奥拉帕利是一种高效的PARP抑制剂,可提高CCA细胞的放射敏感性,且放射增敏程度与奥拉帕利剂量呈正相关,奥拉帕利通过抑制PARP-1、诱导DNA损伤和细胞凋亡以增强辐射效应[50]。因此,PARP抑制剂与其他药物联合治疗IDH突变型肿瘤,可改善患者预后。
Bcl-2抑制剂:IDH1/2突变的原代人AML细胞比IDH1/2野生型细胞对Bcl-2抑制剂维奈克拉的敏感性更高,目前在血液系统恶性肿瘤的体外和异种移植模型中已开展相关研究[51]。IDH1/2突变型人AML细胞通过抑制2-HG介导的线粒体电子传递链中的细胞色素C氧化酶(cytochrome coxidase,COX)活性,降低线粒体在Bcl-2抑制剂处理后触发细胞凋亡的阈值,从而增加其对Bcl-2抑制剂的治疗敏感性[51]。维奈克拉单药治疗IDH1/2突变的复发/难治性AML患者中,4例完全缓解。维奈克拉和去甲基化药物联合治疗被FDA批准用于不能耐受强化化疗的IDH1/2突变的老年AML患者,现有研究表明维奈克拉和去甲基化药物联合疗法在治疗IDH1/2突变的复发/难治性AML患者中取得了良好疗效,完全缓解或完全缓解伴血液学部分恢复者达50%,mOS为15个月[52]。
4. 小结与展望
IDH1和IDH2突变发生于各种实体瘤和骨髓恶性肿瘤中。IDH突变及2-HG已成为脑胶质瘤、AML、iCCA重要的生物标志物,也是极具希望的治疗靶点。IDH抑制剂可作用于IDH突变位点,使体内致癌性代谢物2-HG减少,同时诱导组蛋白和DNA去甲基化,抑制肿瘤发展。针对IDH突变的靶向药物、免疫治疗有望为IDH突变肿瘤的治疗带来新希望。
作者贡献:林美佳负责文献查阅及撰写论文;黄雄飞负责论文修改指导;曾也婷、王心睿负责论文审校。利益冲突:所有作者均声明不存在利益冲突 -
[1] Waitkus MS, Diplas BH, Yan H. Isocitrate dehydrogenase mutations in glioma[J]. Neuro Oncol, 2016, 18: 16-26. DOI: 10.1093/neuonc/nov136
[2] Biaglow JE, Miller RA. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy[J]. Cancer Biol Ther, 2005, 4: 6-13.
[3] Aykin-Burns N, Ahmad IM, Zhu Y, et al. Increased levels of superoxide and H2O2 mediate the differential suscep-tibility of cancer cells versus normal cells to glucose deprivation[J]. Biochem J, 2009, 418: 29-37. DOI: 10.1042/BJ20081258
[4] Yang B, Zhong C, Peng Y, et al. Molecular mechanisms of "off-on switch" of activities of human IDH1 by tumor-associated mutation R132H[J]. Cell Res, 2010, 20: 1188-1200. DOI: 10.1038/cr.2010.145
[5] Parsons DW, Jones S, Zhang X, et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme[J]. Science, 2008, 321: 1807-1812. DOI: 10.1126/science.1164382
[6] Mardis ER, Ding L, Dooling DJ, et al. Recurring Mutations Found by Sequencing an Acute Myeloid Leukemia Genome[J]. N Engl J Med, 2009, 361: 1058-1066. DOI: 10.1056/NEJMoa0903840
[7] Moeini A, Sia D, Bardeesy N, et al. Molecular Pathogenesis and Targeted Therapies of Intrahepatic Cholangiocarcinoma[J]. Clin Cancer Res, 2016, 22: 291-300. DOI: 10.1158/1078-0432.CCR-14-3296
[8] Tommasini-Ghelfi S, Murnan K, Kouri FM, et al. Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease[J]. Sci Adv, 2019, 5: eaaw4543. DOI: 10.1126/sciadv.aaw4543
[9] Harding JJ, Lowery MA, Shih AH, et al. Isoform Switching as a Mechanism of Acquired Resistance to Mutant Isocitrate Dehydrogenase Inhibition[J]. Cancer Discov, 2018, 8: 1540-1547. DOI: 10.1158/2159-8290.CD-18-0877
[10] Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation[J]. Cancer Cell, 2010, 18: 553-567. DOI: 10.1016/j.ccr.2010.11.015
[11] Sulkowski PL, Corso CD, Robinson ND, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity[J]. Sci Transl Med, 2017, 9: eaaI2463. DOI: 10.1126/scitranslmed.aal2463
[12] Schvartzman JM, Reuter VP, Koche RP, et al. 2-hydroxyglutarate inhibits MyoD-mediated differentiation by prevent-ing H3K9 demethylation[J]. Proc Natl Acad Sci USA, 2019, 116: 12851-12856. DOI: 10.1073/pnas.1817662116
[13] Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults[J]. Lancet, 2018, 392: 432-446. DOI: 10.1016/S0140-6736(18)30990-5
[14] Su YT, Phan FP, Wu J. Perspectives on IDH Mutation in Diffuse Gliomas[J]. Trends Cancer, 2018, 4: 605-607. DOI: 10.1016/j.trecan.2018.06.006
[15] Huang RY, Young RJ, Ellingson BM, et al. Volumetric analysis of IDH-mutant lower-grade glioma: a natural history study of tumor growth rates before and after treatment[J]. Neuro Oncol, 2020, 22: 1822-1830. DOI: 10.1093/neuonc/noaa105
[16] Molenaar RJ, Radivoyevitch T, Nagata Y, et al. IDH1/2 Mutations Sensitize Acute Myeloid Leukemia to PARP Inhibition and This Is Reversed by IDH1/2-Mutant Inhibitors[J]. Clin Cancer Res, 2018, 24: 1705-1715. DOI: 10.1158/1078-0432.CCR-17-2796
[17] Xu Q, Li Y, Lv N, et al. Correlation between isocitrate dehydrogenase gene aberrations and prognosis of patients with acute myeloid leukemia: a systematic review and meta-analysis[J]. Clin Cancer Res, 2017, 23: 4511-4522. DOI: 10.1158/1078-0432.CCR-16-2628
[18] Haga H, Patel T. Molecular diagnosis of intrahepatic cholangiocarcinoma[J]. J Hepatobiliary Pancreat Sci, 2015, 22: 114-123. DOI: 10.1002/jhbp.156
[19] Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the US: Intrahepatic Disease on the Rise[J]. Oncologist, 2016, 21: 594-599. DOI: 10.1634/theoncologist.2015-0446
[20] Bai X, Zhang H, Zhou Y, et al. Ten-Eleven Translocation 1 Promotes Malignant Progression of Cholangiocarcinoma With Wild-Type Isocitrate Dehydrogenase 1[J]. Hepatology, 2021, 73: 1747-1763. DOI: 10.1002/hep.31486
[21] Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas[J]. Oncogene, 2013, 32: 3091-3100. DOI: 10.1038/onc.2012.315
[22] Tateishi K, Wakimoto H, Iafrate AJ, et al. Extreme Vulnerability of IDH1 Mutant Cancers to NAD+ Depletion[J]. Cancer Cell, 2015, 28: 773-784. DOI: 10.1016/j.ccell.2015.11.006
[23] Nagashima H, Lee CK, Tateishi K, et al. Poly(ADP-ribose) Glycohydrolase Inhibition Sequesters NAD(+) to Potentiate the Metabolic Lethality of Alkylating Chemo-therapy in IDH-Mutant Tumor Cells[J]. Cancer Discov, 2020, 10: 1672-1689. DOI: 10.1158/2159-8290.CD-20-0226
[24] McDuff SGR, Dietrich J, Atkins KM, et al. Radiation and chemotherapy for high-risk lower grade gliomas: Choosing between temozolomide and PCV[J]. Cancer Med, 2020, 9: 3-11. DOI: 10.1002/cam4.2686
[25] Yamashita AS, da Costa Rosa M, Borodovsky A, et al. Demethylation and epigenetic modification with 5-azacytidine reduces IDH1 mutant glioma growth in combination with temozolomide[J]. Neuro Oncol, 2019, 21: 189-200. DOI: 10.1093/neuonc/noy146
[26] Raulet DH. Roles of the NKG2D immunoreceptor and its ligands[J]. Nat Rev Immunol, 2003, 3: 781-790. DOI: 10.1038/nri1199
[27] Zhang X, Kim WJ, Rao AV, et al. In vivo efficacy of decitabine as a natural killer cell-mediated immunotherapy against isocitrate dehydrogenase mutant gliomas[J]. Neurosurg Focus, 2022, 52: E3.
[28] DiNardo CD, Stein AS, Stein EM, et al. Mutant Isocitrate Dehydrogenase 1 Inhibitor Ivosidenib in Combination With Azacitidine for Newly Diagnosed Acute Myeloid Leukemia[J]. J Clin Oncol, 2021, 39: 57-65.
[29] Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med, 2010, 362: 1273-1281. DOI: 10.1056/NEJMoa0908721
[30] Yang X, Wang J. Precision therapy for acute myeloid leukemia[J]. J Hematol Oncol, 2018, 11: 3. DOI: 10.1186/s13045-017-0543-7
[31] Wang SS, Bandopadhayay P, Jenkins MR. Towards Immunotherapy for Pediatric Brain Tumors[J]. Trends Immunol, 2019, 40: 748-761. DOI: 10.1016/j.it.2019.05.009
[32] Roerden M, Nelde A, Walz JS. Neoantigens in Hematolo-gical Malignancies-Ultimate Targets for Immunotherapy?[J]. Front Immunol, 2019, 10: 3004. DOI: 10.3389/fimmu.2019.03004
[33] Platten M, Bunse L, Wick A, et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma[J]. Nature, 2021, 592: 463-468. DOI: 10.1038/s41586-021-03363-z
[34] Kohanbash G, Carrera DA, Shrivastav S, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas[J]. J Clin Invest, 2017, 127: 1425-1437. DOI: 10.1172/JCI90644
[35] Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate[J]. Nat Med, 2018, 24: 1192-1203. DOI: 10.1038/s41591-018-0095-6
[36] DiNardo CD, Stein EM, de Botton S, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML[J]. N Engl J Med, 2018, 378: 2386-2398. DOI: 10.1056/NEJMoa1716984
[37] Mellinghoff IK, Ellingson BM, Touat M, et al. Ivosidenib in Isocitrate Dehydrogenase 1-Mutated Advanced Glioma[J]. J Clin Oncol, 2020, 38: 3398-3406. DOI: 10.1200/JCO.19.03327
[38] Andronesi OC, Arrillaga-Romany IC, Ly KI, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate[J]. Nat Commun, 2018, 9: 1474. DOI: 10.1038/s41467-018-03905-6
[39] Lowery MA, Burris HA 3rd, Janku F, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study[J]. Lancet Gastroenterol Hepatol, 2019, 4: 711-720. DOI: 10.1016/S2468-1253(19)30189-X
[40] Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study[J]. Lancet Oncol, 2020, 21: 796-807. DOI: 10.1016/S1470-2045(20)30157-1
[41] Cho YS, Levell JR, Liu G, et al. Discovery and Evaluation of Clinical Candidate IDH305, a Brain Penetrant Mutant IDH1 Inhibitor[J]. ACS Med Chem Lett, 2017, 8: 1116-1121. DOI: 10.1021/acsmedchemlett.7b00342
[42] Chaturvedi A, Herbst L, Pusch S, et al. Pan-mutant-IDH1 inhibitor BAY1436032 is highly effective against human IDH1 mutant acute myeloid leukemia in vivo[J]. Leukemia, 2017, 31: 2020-2028. DOI: 10.1038/leu.2017.46
[43] Fathi AT, DiNardo CD, Kline I, et al. Differentiation Syndrome Associated With Enasidenib, a Selective Inhibitor of Mutant Isocitrate Dehydrogenase 2: Analysis of a Phase 1/2 Study[J]. JAMA Oncol, 2018, 4: 1106-1110. DOI: 10.1001/jamaoncol.2017.4695
[44] Pollyea DA, Tallman MS, de Botton S, et al. Enasi-denib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia[J]. Leukemia, 2019, 33: 2575-2584. DOI: 10.1038/s41375-019-0472-2
[45] Stein EM, DiNardo CD, Fathi AT, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib[J]. Blood, 2019, 133: 676-687. DOI: 10.1182/blood-2018-08-869008
[46] Mellinghoff IK, Penas-Prado M, Peters KB, et al. Vorasidenib, a Dual Inhibitor of Mutant IDH1/2, in Recurrent or Progressive Glioma; Results of a First-in-Human Phase I Trial[J]. Clin Cancer Res, 2021, 27: 4491-4499. DOI: 10.1158/1078-0432.CCR-21-0611
[47] Konteatis Z, Artin E, Nicolay B, et al. Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma[J]. ACS Med Chem Lett, 2020, 11: 101-107. DOI: 10.1021/acsmedchemlett.9b00509
[48] Karpel-Massler G, Nguyen TTT, Shang E, et al. Novel IDH1-Targeted Glioma Therapies[J]. CNS Drugs, 2019, 33: 1155-1166. DOI: 10.1007/s40263-019-00684-6
[49] Fritz C, Portwood SM, Przespolewski A, et al. PARP goes the weasel! Emerging role of PARP inhibitors in acute leukemias[J]. Blood Rev, 2021, 45: 100696. DOI: 10.1016/j.blre.2020.100696
[50] Mao Y, Huang X, Shuang Z, et al. PARP inhibitor olaparib sensitizes cholangiocarcinoma cells to radiation[J]. Cancer Med, 2018, 7: 1285-1296. DOI: 10.1002/cam4.1318
[51] Chan SM, Thomas D, Corces-Zimmerman MR, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia[J]. Nat Med, 2015, 21: 178-184. DOI: 10.1038/nm.3788
[52] Morsia E, McCullough K, Joshi M, et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients[J]. Am J Hematol, 2020, 95: 1511-1521. DOI: 10.1002/ajh.25978
-
期刊类型引用(2)
1. 于家琪,魏秀丽. 多药联合治疗老年急性髓系白血病1例并文献复习. 中国现代医生. 2024(09): 128-131 .  百度学术
百度学术
2. 赵濛,任彦博,杜瑞平,宋利文,贺志雄,高民. 水飞蓟粕替代豆粕对羔羊生长性能、血液生化指标及肝脏抗氧化指标和代谢相关基因表达的影响. 动物营养学报. 2024(04): 2524-2540 .  百度学术
百度学术
其他类型引用(2)

 作者投稿
作者投稿 专家审稿
专家审稿 编辑办公
编辑办公 邮件订阅
邮件订阅 RSS
RSS

 下载:
下载:











