Expert Consensus on Rapid SARS-CoV-2 Antigen Testing(2022)
More Information-
摘要: 新型冠状病毒肺炎疫情持续时间长、流行范围广,给全球公共卫生带来了巨大负担。作为感染早期诊断和疫情防控的主要检测手段之一,新型冠状病毒抗原快速检测已在国内逐步开展临床应用。就目前医务人员和社会公众所关注的新型冠状病毒抗原检测相关问题,中国医院协会临床微生物实验室专业委员会组织医学检验、临床医学、感染控制、公共卫生及体外诊断产品研发等多领域专家,依据国内外最新研究进展和应用实践,共同制定了《新型冠状病毒抗原快速检测专家共识(2022)》。本共识介绍了目前新型冠状病毒抗原快速检测方法的技术原理、性能特点、结果解读及处置建议,并就不同场景下新型冠状病毒抗原快速检测应用策略和注意事项进行了解析,以期为临床诊疗和疫情防控中正确理解和应用这一检测技术提供参考建议。Abstract: The epidemic of the highly contagious, long lasting and widely popular coronavirus disease 2019 (COVID-19) has imposed a huge burden to the global public health. As one of the key methods for early diagnosis of COVID-19 infection, rapid acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen testing has been gradually applied in China. To address concerns raised by both health care workers and the public, based on the latest research and clinical practices, the Sub-committee of Clinical Microbiology Laboratory, Chinese Hospital Association proposed Expert Consensus on Rapid SARS-CoV-2 Antigen Testing (2022). The consensus panel is composed of experts from multiple disciplines, including laboratory medicine, clinical medicine, infection control, public health, research and development of in vitro diagnostic products. The consensus describes its principle, technological characteristics, results interpretation and disposal recommendations, and analyzes the strategies and matters needing attention in different application scenarios. We expect the consensus to help correct understanding and application of rapid SARS-CoV-2 antigen testing in the diagnosis, treatment, prevention, and control of COVID-19.作者贡献:徐英春牵头制订共识框架,组建共识制订工作组,组织工作组讨论、修订并审阅定稿;胡继红组织工作组复习文献,起草、修订共识并审阅定稿;所有成员参与讨论、修订并形成共识意见;王瑶、何书宇、邹嘉琪对共识进行撰写、修订和审校。利益冲突:所有参与共识制订的人员均声明不存在利益冲突编审组 (按姓氏首字母排序):阿祥仁(青海省人民医院),褚云卓(中国医科大学附属第一医院),多丽波(哈尔滨医科大学附属第二医院),高春海(临沂市人民医院),顾兵(广东省人民医院),郭大文(哈尔滨医科大学附属第一医院),郭鹰(重庆医科大学附属巴南医院),韩崇旭(江苏省苏北人民医院),侯锐钢(山西医科大学第二医院),胡辛兰(福建省立医院),胡云建(北京医院),贾伟(宁夏医科大学总医院),康梅(四川大学华西医院),柯江维(江西省儿童医院),李彬(福建医科大学附属协和医院),李刚(宁夏医科大学总医院),李俊明(南昌大学第一附属医院),廖康(中山大学附属第一医院),林宁(江苏省淮安市第一人民医院),林勇平(广州医科大学附属第一医院),刘文恩(中南大学湘雅医院),刘小平(北京大学深圳医院),刘勇(中国医科大学附属盛京医院),卢志明(山东第一医科大学附属省立医院山东省立医院),罗春华(湖北省宜昌市中心人民医院),罗春玉(赤峰学院附属医院),罗燕萍(中国药师学会),马小军(中国医学科学院北京协和医院),马筱玲(中国科技大学附属第一医院),毛小琴(云南省第一人民医院),木克代斯·米尔地洋(新疆维吾尔自治区人民医院),穆红(天津市第一中心医院),潘艳(江苏省涟水县人民医院),单斌(昆明医科大学第一附属医院),沈瀚(南京大学附属鼓楼医院),沈继录[安徽省公共卫生临床中心安徽医科大学第一附属医院(北区)],王晶(大连医科大学附属第一医院),魏莲花(甘肃省人民医院检验中心),吴洁(中国医学科学院北京协和医院),谢丽(广西医科大学第二附属医院),徐雪松(吉林大学中日联谊医院),杨滨(福建医科大学附属第一医院),杨青(浙江大学医学院附属第一医院),喻华(四川省医学科学院四川省人民医院),张利侠(陕西省人民医院),张义(山东大学齐鲁医院),张樱(解放军总医院第一医学中心),赵建宏(河北医科大学第二医院河北省临床检验中心),周泽奇[丹娜(天津)生物科技股份有限公司],朱镭(山西省儿童医院)外审组 (按姓氏首字母排序):曹东林(广东省第二人民医院),陈浪(北京金沃夫生物工程科技有限公司),戴二黑(石家庄市第五医院),戴俊(广州海关技术中心),段朝晖(中山大学孙逸仙纪念医院),关伟杰(广州医科大学附属第一医院广州呼吸健康研究院),郝晓珂(西安区域医学检验中心),胡凤玉(广州市第八人民医院),李六亿(北京大学第一医院),李一荣(武汉大学中南医院),梁皓钧(香港大学公共卫生学院),马学军(中国疾病预防控制中心病毒病预防控制所),秦晓松(中国医科大学附属盛京医院),苏建荣(首都医科大学附属北京友谊医院),汤一苇(丹纳赫诊断平台/赛沛中国),陶志华(浙江大学医学院附属第二医院),王华梁(上海市实验医学研究院),王云峰(美国亚特兰大格雷迪纪念医院),张国军(首都医科大学附属北京天坛医院),赵锐(北京电力医院),郑磊(南方医科大学南方医院),周海卫(中国食品药品检定研究院),卓超(广州医科大学附属第一医院)秘书组 (按姓氏首字母排序):何书宇(广州万孚生物技术股份有限公司),卢国萍[梅里埃诊断产品(上海)有限公司],汪小芳(广州万孚生物技术股份有限公司),杨文航(中国医学科学院北京协和医院),杨洋(中国医学科学院北京协和医院),张戈(中国医学科学院北京协和医院),邹嘉琪(广州万孚生物技术股份有限公司)执笔人:邹嘉琪(广州万孚生物技术股份有限公司),王瑶(中国医学科学院北京协和医院),康可人(广州万孚生物技术股份有限公司),胡继红(国家老年医学中心中国医学科学院老年医学研究院北京医院),徐英春(中国医学科学院北京协和医院)
-
表 1 常用新型冠状病毒抗原快速检测方法及技术特点
检测方法 标记物 技术特点 适用场景 胶体金免疫层析法 胶体金颗粒 优点:无需借助仪器,目视即可判读结果
缺点:可能存在个体主观判断的差异基层医疗卫生机构、隔离观察人员及社区居民自测 乳胶免疫层析法 乳胶微球 荧光免疫层析法 荧光微球 优点:稳定性好、不易受自然光的干扰,敏感性高
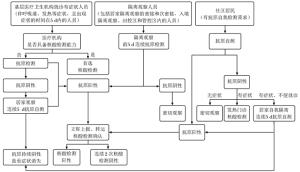
缺点:需免疫分析仪辅助基层医疗卫生机构 临床症状 抗原检测 核酸检测 抗体检测* 结果解读 处置建议 IgM IgG 无症状 - - - - 无感染证据 NA - - - + 已接种疫苗,或既往感染 NA - - + -/+ 疫苗接种早期,或无症状感染者/病毒携带者核酸检测转阴后 (1)无接触史者:自我观察
(2)有接触史者:不排除处于感染潜伏期,按疫情防控政策隔离、医学观察、核酸检测,隔离前5 d连续进行抗原检测+ - -/+ -/+ 感染早期,或抗原非特异性反应 立即进行核酸检测,按疫情防控政策要求进行隔离、医学观察;连续两次核酸检测阴性(至少间隔24 h)可排除疑似病例的诊断 -/+ + -/+ -/+ 无症状感染者或病毒携带者 立即上报,按疫情防控政策要求进行集中隔离、密切医学观察并采取相关措施 有症状 -
-
--
-
--
-
+-
+
-/+检测窗口期,或其他疾病
感染恢复期,或既往感染史,或已接种疫苗,或其他疾病
感染早期,或已接种疫苗,或其他疾病(1)无接触史者:发热门诊就诊、核酸检测,鉴别诊断、对症治疗;不便就诊者居家隔离并连续5 d抗原自测,至抗原持续阴性且症状消失
(2)有接触史者:按疫情防控政策要求进行隔离、核酸检测、鉴别诊断、对症治疗,隔离前5 d连续进行抗原检测+ - -/+ -/+ 感染早期,或其他疾病发生抗原非特异性反应 立即进行核酸检测,按疫情防控政策要求进行隔离、鉴别诊断、对症治疗,连续2次核酸阴性(至少间隔24 h)可排除疑似病例诊断 -/+ + -/+ -/+ 确诊病例 立即上报,按疫情防控政策转送定点医院治疗 *排除抗体非特异性交叉反应;NA: 无处置建议 -
[1] 国家卫生健康委员会办公厅, 国家中医药管理局办公室. 关于印发新型冠状病毒肺炎诊疗方案(试行第九版)的通知(国卫办医函〔2022〕71号)[EB/OL]. (2022-03-14)[2022-04-10]. http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm. [2] 国务院应对新型冠状病毒肺炎疫情联防联控机制综合组. 关于印发新冠病毒抗原检测应用方案(试行)的通知(联防联控机制综发〔2022〕21号)[EB/OL]. (2022-03-11)[2022-04-10]. http://www.nhc.gov.cn/yzygj/s7659/202203/d4d7fb72088447f7a4f9cd10966a67eb.shtml. [3] 医政医管局. 新冠病毒抗原检测应用方案(试行)政策解读[EB/OL]. (2022-03-11)[2022-04-10]. http://www.nhc.gov.cn/yzygj/s7659/202203/4573dfb9cca244509c29b964ba287889.shtml. [4] 国家药品监督管理局. 医疗器械数据查询[EB/OL]. (2022-04-07) [2022-04-10]. https://www.nmpa.gov.cn/datasearch/search-result.html. [5] World Health Organization. WHO Emergency Use Listing for in vitro diagnostics (IVDs) detecting SARS-CoV-2[EB/OL]. (2022-02-23)[2022-04-10]. https://extranet.who.int/pqweb/sites/default/files/documents/220223_EUL_SARS-CoV-2_product_list.pdf. [6] U.S. Food & Drug Administration. In Vitro Diagnostics EUAs-Antigen Diagnostic Tests for SARS-CoV-2[EB/OL]. (2022-04-07)[2022-04-10]. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-anti-gen-diagnostic-tests-sars-cov-2. [7] European Commission Directorate-General for Health and Food Safety. EU health preparedness: A common list of COVID-19 rapid antigen tests; A common standardised set of data to be included in COVID-19 test result certificates; and A common list of COVID-19 laboratory based antigenic assays[EB/OL]. (2022-03-30)[2022-04-10]. https://ec.europa.eu/health/system/files/2022-03/covid-19_rat_common-list_en_1.pdf. [8] 范曼如, 申泉, 王丹琦, 等. 临床实践指南制订方法: 形成推荐意见的共识方法学[J]. 中国循证心血管医学杂志, 2019, 11: 647-653. doi: 10.3969/j.issn.1674-4055.2019.06.02 [9] 北京协和医院罕见病多学科协作组, 中国罕见病联盟. 多准则决策分析应用于罕见病药品临床综合评价的专家共识(2022)[J]. 协和医学杂志, 2022, 13: 126-145. https://www.cnki.com.cn/Article/CJFDTOTAL-XHYX202202010.htm [10] Kandimalla R, John A, Abburi C, et al. Current Status of Multiple Drug Molecules, and Vaccines: An Update in SARS-CoV-2 Therapeutics[J]. Mol Neurobiol, 2020, 57: 4106-4116. doi: 10.1007/s12035-020-02022-0 [11] Zandi M, Soltani S. Hemagglutinin-esterase cannot be considered as a candidate for designing drug against COVID-19[J]. Mol Divers, 2021, 25: 1999-2000. doi: 10.1007/s11030-021-10272-w [12] Scheiblauer H, Filomena A, Nitsche A, et al. Comparative sensitivity evaluation for 122 CE-marked rapid diagnostic tests for SARS-CoV-2 antigen, Germany, September 2020 to April 2021[J]. Euro Surveill, 2021, 26: 2100441. [13] Peng Y, Du N, Lei Y, et al. Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design[J]. EMBO J, 2020, 39: e105938. [14] 世界卫生组织. 抗原检测用于诊断SARS-CoV-2感染: 临时指导文件[EB/OL]. (2021-10-06)[2022-04-10]. https://apps.who.int/iris/bitstream/handle/10665/345948/WHO-2019-nCoV-Antigen-Detection-2021.1-chi.pdf. [15] 刘巨钊, 杨玉萍, 徐建波, 等. 新冠病毒S蛋白RBD突变侵染性增强潜在分子作用机制[J/OL]. 生物学杂志, 2022: 1-8. [2022-04-09]. http://kns.cnki.net/kcms/detail/34.1081.q.20220321.1514.002.html. [16] 徐本锦, 范蕾, 杜淼, 等. 新冠病毒核衣壳蛋白结构与功能的生物信息学分析及原核表达[J/OL]. 中国免疫学杂志, 2021: 1-25. http://kns.cnki.net/kcms/detail/22.1126.R.20210924.0101.002.html. [17] Bates TA, Weinstein JB, Farley S, et al. Cross-reactivity of SARS-CoV structural protein antibodies against SARS-CoV-2[J]. Cell Rep, 2021, 34: 108737. doi: 10.1016/j.celrep.2021.108737 [18] Corman VM, Haage VC, Bleicker T, et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study[J]. Lancet Microbe, 2021, 2: e311-e319. doi: 10.1016/S2666-5247(21)00056-2 [19] Seo G, Lee G, Kim MJ, et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyn-geal Swab Specimens Using Field-Effect Transistor-Based Biosensor[J]. ACS Nano, 2020, 14: 5135-5142. doi: 10.1021/acsnano.0c02823 [20] Mahari S, Roberts A, Shahdeo D, et al. eCovSens-Ultrasensitive Novel In-House Built Printed Circuit Board Based Electrochemical Device for Rapid Detection of nCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2[J]. bioRxiv, 2020. doi: 10.1101/2020.04.24.059204. [21] Dinnes J, Deeks JJ, Berhane S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection[J]. Cochrane Database Syst Rev, 2021, 3: CD013705. [22] Bruzzone B, De Pace V, Caligiuri P, et al. Comparative diagnostic performance of rapid antigen detection tests for COVID-19 in a hospital setting[J]. Int J Infect Dis, 2021, 107: 215-218. doi: 10.1016/j.ijid.2021.04.072 [23] Chaimayo C, Kaewnaphan B, Tanlieng N, et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand[J]. Virol J, 2020, 17: 177. doi: 10.1186/s12985-020-01452-5 [24] Brümmer LE, Katzenschlager S, Gaeddert M, et al. Accur-acy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis[J]. PLoS Med, 2021, 18: e1003735. doi: 10.1371/journal.pmed.1003735 [25] Smith RL, Gibson LL, Martinez PP, et al. Longitudinal Assessment of Diagnostic Test Performance Over the Course of Acute SARS-CoV-2 Infection[J]. J Infect Dis, 2021, 224: 976-982. doi: 10.1093/infdis/jiab337 [26] Khalid MF, Selvam K, Jeffry A, et al. Performance of Rapid Antigen Tests for COVID-19 Diagnosis: A Systematic Review and Meta-Analysis[J]. Diagnostics (Basel), 2022, 12: 110. doi: 10.3390/diagnostics12010110 [27] Deng Q, Ye G, Pan Y, et al. High Performance of SARS-Cov-2N Protein Antigen Chemiluminescence Immunoassay as Frontline Testing for Acute Phase COVID-19 Diagnosis: A Retrospective Cohort Study[J]. Front Med (Lausanne), 2021, 8: 676560. [28] Yokoyama R, Kurano M, Nakano Y, et al. Association of the Serum Levels of the Nucleocapsid Antigen of SARS-CoV-2 With the Diagnosis, Disease Severity, and Antibody Titers in Patients With COVID-19: A Retrospective Cross-Sectional Study[J]. Front Microbiol, 2021, 12: 791489. doi: 10.3389/fmicb.2021.791489 [29] European Centre for Disease Prevention and Control. Options for the use of rapid antigen tests for COVID-19 in the EU/EEA-first update[EB/OL]. (2021-10-26)[2022-04-09]. https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-first-update. [30] Jungnick S, Hobmaier B, Mautner L, et al. Detection of the new SARS-CoV-2 variants of concern B. 1.1.7 and B. 1.351 in five SARS-CoV-2 rapid antigen tests (RATs), Germany, March 2021[J]. Euro Surveill, 2021, 26: 2100413. [31] Pulliam J, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa[J]. Science, 2022, 376: eabn4947. doi: 10.1126/science.abn4947 [32] Saxena SK, Kumar S, Ansari S, et al. Characterization of the novel SARS-CoV-2 Omicron (B. 1.1.529) variant of concern and its global perspective[J]. J Med Virol, 2022, 94: 1738-1744. doi: 10.1002/jmv.27524 [33] Soni A, Herbert C, Filippaios A, et al. Comparison of Rapid Antigen Tests' Performance between Delta (B. 1.61.7; AY. X) and Omicron (B. 1.1.529; BA1) Variants of SARS-CoV-2: Secondary Analysis from a Serial Home Self-Testing Study[J]. medRxiv, 2022. doi: 10.1101/2022.02.27.22271090. [34] Bekliz M, Perez-Rodriguez F, Puhach O, et al. Sensitivity of SARS-CoV-2 antigen-detecting rapid tests for Omicron variant[J]. medRxiv, 2021. doi: https://www.medrxiv.org/content/10.1101/2021.12.18.21268018. [35] Deerain J, Druce J, Tran T, et al. Assessment of the Analytical Sensitivity of 10 Lateral Flow Devices against the SARS-CoV-2 Omicron Variant[J]. J Clin Microbiol, 2022, 60: e247921. [36] Bullard J, Dust K, Funk D, et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples[J]. Clin Infect Dis, 2020, 71: 2663-2666. doi: 10.1093/cid/ciaa638 [37] U.S. FOOD & DRUG ADMINISTRATION. Potential for False Positive Results with Antigen Tests for Rapid Detection of SARS-CoV-2-Letter to Clinical Laboratory Staff and Health Care Providers[EB/OL]. (2020-11-03)[2022-04-10]. https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-positive-results-antigen-tests-rapid-detection-sars-cov-2-letter-clinical-laboratory. [38] Mouliou DS, Gourgoulianis KI. False-positive and false-negative COVID-19 cases: respiratory prevention and management strategies, vaccination, and further perspectives[J]. Expert Rev Respir Med, 2021, 15(8): 993-1002. doi: 10.1080/17476348.2021.1917389 [39] Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening[J]. Sci Adv, 2021, 7: eabd5393. doi: 10.1126/sciadv.abd5393 [40] Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 Test Sensitivity-A Strategy for Containment[J]. N Engl J Med, 2020, 38: e120. [41] 国务院应对新型冠状病毒肺炎疫情联防联控机制综合组. 基层医疗卫生机构新冠病毒抗原检测基本要求及流程[EB/OL]. (2022-03-10)[2022-04-10]. http://www.nhc.gov.cn/yzygj/s7659/202203/d4d7fb72088447f7a4f9cd10966a67eb/files/b62d9eeeca8242b3a22e78a25ef9fd92.pdf. [42] 中国合格评定国家认可委员会. 2019临床免疫学定性检验程序性能验证指南: CNAS-GL038[S]. 2019. [43] 国务院应对新型冠状病毒肺炎疫情联防联控机制综合组. 新冠病毒抗原自测基本要求及流程[EB/OL]. (2022-03-10)[2022-04-10]. http://www.nhc.gov.cn/yzygj/s7659/202203/d4d7fb72088447f7a4f9cd10966a67eb/files/8db5dd22ccb84b6296bc4257ba7db9c0.pdf. [44] Stohr J, Zwart VF, Goderski G, et al. Self-testing for the detection of SARS-CoV-2 infection with rapid antigen tests for people with suspected COVID-19 in the community[J]. Clin Microbiol Infect, 2021, S1198-743X(21)00434-1. doi: 10.1016/j.cmi.2021.07.039. [45] Maya S, Kahn JG. Cost-effectiveness of antigen testing for ending COVID-19 isolation[J]. medRxiv, 2022, 2022.03.21.22272687. doi: 10.1101/2022.03.21.22272687. [46] 交通运输部, 外交部, 海关总署. 关于做好国际航行船舶船员新冠肺炎疫情远端防控的公告(交通运输部公告2022年第14号)[EB/OL]. (2022-01-28)[2022-04-10]. http://www.gov.cn/zhengce/zhengceku/2022-02/13/content_5673345.htm. [47] 天津市卫生健康委员会. 市卫生健康委转发市防控指挥部关于印发天津市新冠病毒抗原检测应用阶段性实施方案(试行)的通知(津卫医政〔2022〕151号)[EB/OL]. (2022-03-29)[2022-04-10]. http://wsjk.tj.gov.cn/ZWGK3158/ZCFG6243_1/GZWJ625/202203/t20220329_5842885.html. -


 作者投稿
作者投稿 专家审稿
专家审稿 编辑办公
编辑办公 邮件订阅
邮件订阅 RSS
RSS

 下载:
下载:













