Status and Prospect of Clinical Application of the Evaluation of Anti-Ⅹa Activity in Anticoagulant Therapy
-
摘要: 随着检测技术的进步,临床中各种疾病伴随血栓的检出率越来越高。普通肝素和低分子量肝素是目前临床应用较多的注射用抗凝剂,其中普通肝素的半衰期短、无肾毒性、有拮抗剂;低分子量肝素半衰期较长,需在一些特殊人群如儿童、孕妇、老人中进行监测。口服抗凝剂中,除华法林等传统药物外,靶向活化凝血因子Ⅹa的抗凝药物如利伐沙班亦越来越多地应用于临床。既往采用活化部分凝血活酶时间对普通肝素进行监测,而对低分子量肝素、新型抗Ⅹa类药物监测手段和监测意识不强。随着人们对抗凝治疗认识的不断深入,抗Ⅹa活性检测作为一种新的抗凝监测手段,临床应用越来越广泛,但目前检测结果很难与临床表现相关联,未来仍需大型随机对照试验加以验证。
-
关键词:
- 抗Ⅹa活性检测 /
- 肝素 /
- 低分子量肝素 /
- 活化部分凝血活酶时间 /
- 抗凝
Abstract: With the progress of detection technology, the incidence rate of thrombosis in various diseases is increasing. Unfractionated heparin (UFH) and low molecular weight heparin (LMWH) are commonly used anticoagulants in the clinic. UFH has a short half-life, no nephrotoxicity, and antagonists. LMWH has a long half-life, and only needs to be monitored in some special persons, such as children, pregnant women, and the elderly people. At present, among oral anticoagulants, in addition to warfarin and other traditional oral anticoagulants, there are also many anticoagulants targeting factor Ⅹa, such as rivaroxaban. More and more attention has been paid to the monitoring of anticoagulant therapy. Activated partial thromboplastin time was used to monitor heparin in the past, but LMWH and new anti-Ⅹa anticoagulants are not well monitored. With the deepening understanding of anticoagulant therapy, anti-Ⅹa as a monitoring means can be used to monitor anti-Ⅹa drugs, and the scope of use is wider and wider. However, due to the lack of large-scale randomized controlled trials, it is difficult to correlate anti-Ⅹa activity with clinical manifestations.作者贡献:邸平负责查阅文献,撰写初稿;李绵洋负责文章构思,提出修改意见。利益冲突:无 -
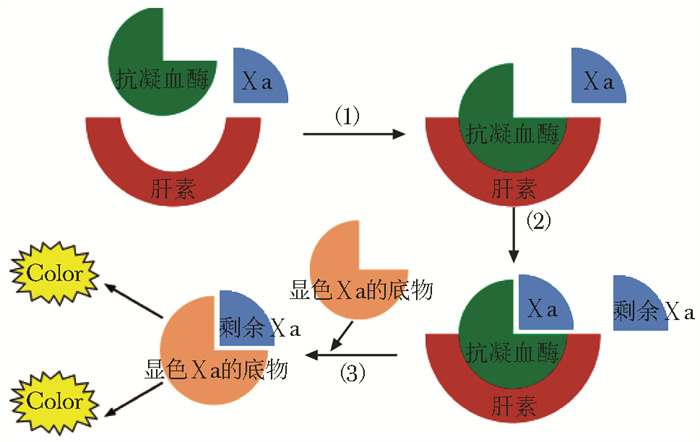
图 1 抗Ⅹa活性检测原理[1]
表 1 利伐沙班临床适应证及抗Ⅹa监测范围[18]
适应证 剂量 谷值(范围),μg/L 峰值(范围),μg/L 全髋关节置换术后VTE预防 10 mg,1次/d 8(1~38) 125(91~196) DVT治疗 20 mg,1次/d 26(6~87) 270(189~419) 非瓣膜性房颤(肌酐清除率≥50 mL/min) 20 mg,1次/d 44(12~137) 249(184~343) 非瓣膜性房颤(肌酐清除率30~49 mL/min) 15 mg,1次/d 57(18~136) 229(178~313) 急性冠脉综合征的二级预防 2.5 mg,2次/d 17(6~37) 46(28~70) VTE:静脉血栓栓塞症;DVT:深静脉血栓 表 2 抗凝药物监测指征及监测项目
抗凝药物 监测指征 监测项目 普通肝素 常规监测 APTT、ACT、抗Ⅹa 低分子量肝素 妊娠期、严重肾功能不全、严重出血风险、肥胖或体质量过低等 抗Ⅹa 利伐沙班 有影响药物代谢的因素,如肾肝功能不全、肥胖或体质量过低、胃肠道吸收不良等 抗Ⅹa、PT APTT:活化部分凝血活酶时间;ACT:激活凝血时间;PT: 凝血酶原时间 -
[1] 滕媛, 李勇男, 楼松, 等. 抗Ⅹa活性在普通肝素抗凝监测中应用的研究进展[J]. 中国体外循环杂志, 2018, 16: 248-251. https://www.cnki.com.cn/Article/CJFDTOTAL-TWXH201804016.htm [2] Kovács B, Bereczky Z, Selmeczi A, et al. Progressive chromogenic anti-factor Ⅹa assay and its use in the classification of antithrombin deficiencies[J]. Clin Chem Lab Med, 2014, 52: 1797-1806. http://www.ncbi.nlm.nih.gov/pubmed/24968404 [3] David A, Trevor P, Jeffrey I, et al. Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [J]. Chest, 2018, 141: e24S-e43S. http://www.scienceopen.com/document?vid=641686e2-5321-45a5-b505-07883517eee5 [4] Khan F, Tritschler T, Kahn SR, et al. Venous thromboembolism[J]. Lancet, 2021, 398: 64-77. doi: 10.1016/S0140-6736(20)32658-1 [5] Mulloy B, Hogwood J, Gray E, et al. Pharmacology of Heparin and Related Drugs[J]. Pharmacol Rev, 2016, 68: 76-141. http://www.ncbi.nlm.nih.gov/pubmed/26672027 [6] Hannah L, Leah M, Majed A, et al. Updates in Anticoagulation Therapy Monitoring[J]. Blood, 1992, 79: 1-17. doi: 10.1182/blood.V79.1.1.1 [7] Van Cott EM, Roberts AJ, Dager WE. Laboratory Monitor-ing of Parenteral Direct Thrombin Inhibitors[J]. Semin Thromb Hemost, 2017, 43: 270-276. doi: 10.1055/s-0036-1597297 [8] Zhang N, Lou W, Ji F, et al. Low molecular weight heparin and cancer survival: clinical trials and experimental mechanisms[J]. Cancer Res Clin Oncol, 2016, 142: 1807-1816. doi: 10.1007/s00432-016-2131-6 [9] Bates S, Weitz J, Johnston M, et al. Use of a fixed activated partial thromboplastin time ratio to establish a therapeutic range for unfractionated heparin[J]. Arch Intern Med, 2001, 161: 385-391. doi: 10.1001/archinte.161.3.385 [10] Byun JH, Jang IS, Kim JW, et al. Establishing the heparin therapeutic range using aPTT and anti-Ⅹa measurements for monitoring unfractionated heparin therapy[J]. Blood Res, 2016, 51: 171-174. doi: 10.5045/br.2016.51.3.171 [11] Kumano O, Akatsuchi K, Amiral J, et al. Updates on Anticoagulation and Laboratory Tools for Therapy Monitoring of Heparin, Vitamin K Antagonists and Direct Oral Anticoagulants[J]. Biomedicines, 2021, 9: 264-269. doi: 10.3390/biomedicines9030264 [12] Vandiver JW, Vondracek TG. Antifactor Ⅹa levels versus activated partial thromboplastin time for monitoring unfractionated heparin[J]. Pharmacotherapy, 2012, 32: 546-558. doi: 10.1002/j.1875-9114.2011.01049.x [13] Tan J, Ning T, Zhang W, et al. Heparin locks with low and high concentration in haemodialysis patients: A systematic review and meta-analysis[J]. Int J Nurs Pract, 2021, 27: e12907. doi: 10.1111/ijn.12907 [14] Arachchillage D, Kamani F, Deplano S, et al. Should we abandon the aPTT for monitoring unfractionated heparin?[J]. Thromb Res, 2017, 8: 157-161. http://www.ncbi.nlm.nih.gov/pubmed/28759760 [15] Alhenc-Gelas M, Jestin-Le Guernic C, Vitoux JF, et al. Adjusted versus fixed doses of the low-molecular weight heparin fragmin in the treatment of deep vein thrombosis. Fragmin-Study Group[J]. Thromb Haemost, 1994, 71: 698-702. doi: 10.1055/s-0038-1642507 [16] Bates S, Greer I, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines[J]. Chest, 2012, 141: e691S-e736S. doi: 10.1378/chest.11-2300 [17] Wong SS, Lau WY, Ng ML, et al. Low-molecular weight heparin infusion as anticoagulation for haemodialysis[J]. Clin Kidney, 2016, 9: 630-635. doi: 10.1093/ckj/sfw049 [18] Meyer MS, Geneviève C, Theodore ES, et al. Laboratory assessment of rivaroxaban: a review[J]. Thromb J, 2013, 11: 11. -


 作者投稿
作者投稿 专家审稿
专家审稿 编辑办公
编辑办公 邮件订阅
邮件订阅 RSS
RSS


 下载:
下载: